Abstract
In recent years, more and more scientific community, food producers, and food industry show increased interest in functional foods containing probiotics, which is a big challenge. The consumption of probiotics in the context of a balanced diet through the consumption of functional foods or through the intake of pharmaceutical preparations has proven to contribute to the improvement of human health, even contributing to the prevention of diseases. In order for probiotics to be considered suitable for consumption, they must contain a minimum concentration of viable cells, namely, at least 107 colony forming units of beneficial microbes per gram. Ensuring the viability of bacterial cells until the moment of consumption is the overriding priority of functional probiotic food manufacturers. Probiotic bacteria are subject to stress conditions not only during food manufacturing but also during gastrointestinal passage, which limit or even compromise their functionality. This paper first examines all the stressful conditions faced by probiotic cells in their production stages and related to the conditions present in the bioreactor fermentation and drying processes as well as factors related to the food matrix and storage. The stress situations faced by probiotic microorganisms during the gastrointestinal transit especially during stomach and intestinal residence are also analyzed. In order to understand the adaptation mechanisms of probiotic bacteria to gastrointestinal stress, intrinsic and adaptive mechanisms identified in probiotic strains in response to acid stress and to bile and bile acid stress are analyzed. In addition, improvement strategies for multiple stress tolerance of lactic acid bacteria through directions dealing with stress, accumulation of metabolites, use of protectants, and regulation of technological parameters are examined. Finally, the definition of postbiotics, inanimate microorganisms and/or their components conferring health benefits, is also introduced. Postbiotics include cell lysates, enzymes, and cell wall fragments derived from probiotic bacteria and may represent an alternative to the use of probiotics, when they do not tolerate stressful conditions.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, the usefulness of the human microbiome for both short-term and long-term human health has been clarified by the scientific community. The microbiome significantly determines the development of the immune system. The role of probiotics has received much scientific attention and is the subject of research in recent decades [1]. Probiotics are living microorganisms that act through competitive exclusion to protect the host from various pathogens and provide nutrients from the breakdown of indigestible dietary carbohydrates, [2, 3] e.g., increasing short-chain fatty acid (SCFA) production [4]. Moreover, symptoms from acute infectious diarrhea, antibiotic-associated diarrhea, Clostridium difficile-associated diarrhea, ulcerative colitis, and irritable bowel syndrome could be treated with probiotics [5]. The administration of probiotics requires either a medical prescription or not, and their composition includes microorganisms that have similarities with the common bacteria found in the gut, most often Bifidobacterium and Lactobacillus spp. that produce lactic acid [5,6,7]. Control of commercial probiotic products can be characterized as insufficient since probiotics are branded and not characterized by the bacterial strain and formulations or manufacturing protocols may be subject to changes over time, dramatically affecting their effectiveness [5].
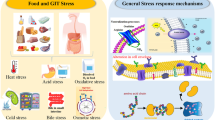
Although there is an inextricable link between the biological activity of probiotics and the beneficial effects on human health through the consumption of foods containing them, this benefit can be degraded due to the stressful conditions faced by the microorganisms from the moment they are produced, until the incorporation in food, their subsequent storage, and consumption [8]. Stress situations faced by probiotic microorganisms during food production may concern the conditions of the fermentation carried out in the bioreactor and preparation of microbial stocks, the drying processes, such as, spray drying and freeze-drying, the food matrix, as well as their storage and characteristics of food [8,9,10].
Among the stress conditions faced by probiotic microorganisms during the gastrointestinal transit are the gastric environment and the intestinal residence. The adaptation mechanisms of probiotic bacteria to gastrointestinal stress are related to the innate or intrinsic and adaptive mechanisms identified in probiotic strains in response to acid stress and to bile and bile acid stress [11,12,13].
The aim of this study is to examine all the factors related to the development of stress throughout the production process of probiotics. The stress conditions that occur both in the stomach environment and in the residence of probiotic cells in the intestinal tract are also examined. Finally, in this study, attention is also focused on the improvement of multiple stress tolerance of lactic acid bacteria (LAB) as these are the microbial strains widely used as probiotics.
Stress Conditions Faced by Probiotic Microorganisms During Food Manufacturing
Nowadays, microorganisms of probiotic properties belong to different classes of microorganisms including yeasts such as Saccharomyces boulardii and Kluyveromyces lactis, Gram-positive non-spore forming bacteria such as those belonging to former Lactobacillus sp. and Bifidobacterium sp., and spore formers such as Bacillus sp.
Recently, Bacillus (B.) sp. such as B. coagulans become very attractive candidates in probiotic industries [14]. This is based not only on the ease of cultivation in higher biomass culture but also on their high resistance to stress conditions; it is easy to keep the high cell and spore number during the whole production chain. It stands out as a property the high temperature resistance of these strains makes industry able to use the many choices of cost-effective methods in downstream processes especially of drying and formulation.
In fact, different types of probiotic yeasts are used nowadays as probiotics in both feed, food, nutraceuticals, and cosmeceutical industries [15]. Yeasts do not have the capacity to produce thermos-resistant intracellular spores. Therefore, they are more sensitive to environmental stresses compared to spore-forming bacteria.
Most of the commercial known formula of probiotic bacteria includes the facultative anaerobic bacteria belonging to Lactobacillus sp. or the strict anaerobic bacteria Bifidobacteria sp. A new classification of Lactobacillus has recently been proposed that includes 23 new genera. The two big groups are non-spore-forming and thus exhibit high sensitivity to environmental stress. Therefore, most of the research related to probiotic protection during the production process is associated with these two groups of bacteria and will therefore be the focus of this review.
Bioreactor Cultivation of Probiotic Microorganisms
The highly diversified groups of probiotic microbes for sure also possess a wide range of nutritional and cultivation condition requirements. However, for all microbes used, the main target is to produce high biomass at the shortest period of time with minimal production cost. During the transfer of production process from small shake flask level to large scale, different parameters are changed. However, large-scale production facilitates the possible improvement of the cultivation process based on the possible control of pH, aeration, and agitation compared to shaking flask. However, large-scale production has also its own problems and most specifically temperature (as cell growth is exothermic reaction in case of aerobic microbes), controlling the oxygen supply to the cells in case of high cell density culture and suppressing foam production in some cultivation processes. However, controlling cultivation conditions and shifting from one cultivation strategy to another are easier in large-scale manufacturing compared to smaller scale.
In addition, it is also necessary to produce cells of some resistance to stresses they are exposed to during downstream processing, storage, and in vivo applications. These include pH, dryness, and osmotic and thermal stresses. Cultivation strategy during scaling up of the process can be designed to improve microbial resistance through both medium formulation and cultivation conditions. The selection and design of the resistance development strategy do not only depend on the type of microorganism but also based on the downstream approach used, product form, and storage conditions of the final product. Some possible strategies to implement during biomass scaling are detailed below.
Adaptation to pH Stress
Adaptation of low pH is one of the main targets during biomass production of probiotics as it improves acid resistance during probiotic in vivo application [16]. Adaptation to pH stress is usually started in small scale as sequential adaptation to low pH before large-scale production. In large-scale production, acid resistance can be achieved by using two-stage continuous fermentation which can enhance multistress of the probiotic strains or using immobilized cultivation system. A recent study also reported that exposure of Bifidobacterium breve cells to multistress conditions (high temperature, low pH, and oxidative stress) during cultivation at very low growth rate can induce high amount of stress proteins to improve multistress conditions during processing and application [17].
Adaptation to Dryness Stress and Osmotic Stresses
Adaptation of cells to dryness stress is usually carried out by successive adaptation process to increase cell viability during processing and storage in dry form especially for non-spore-forming probiotics. This is usually carried out in adaptation to multistress factors such as osmotic stress and pH. The preadaptation strategy to osmotic stress using successive adaptive growth at elevated salt (mainly sodium chloride) and sugar (mainly sucrose) concentrations before production is common for LAB strains such as L. plantarum, L. acidophilus, and L. casei [18].
Production of Microbial Metabolites to Reduce Environmental Stress
Probiotics have the capacity to produce a wide range of bioactive metabolites. Some of these metabolites can play a protectant role against environmental stress such as polysaccharides. For example, production of polysaccharides by LAB can act as natural protective agents to increase cell viability during downstream process. The productivity of polysaccharides by Lactobacillus strains can be controlled by medium composition and cultivation conditions as well [19]. It has been also reported that kefiran production by L. kefiranofaciens in submerged culture can be improved by manipulation of osmotic stress and addition of surfactants [20]. Moreover, addition of carbon dioxide during anerobic probiotic cultivation such as for Bifidobacterium longum not only enhances biomass production but also increases polysaccharide production, which increases cell resistance to stress [21].
Stress Conditions During Drying Processes
In the manufacturing of living probiotic cells, many aspects of these cells are affected such as survival, viability, and growth [22]. The tolerance tests, concerning the selection and evaluation of the strains of probiotic cells, in view of their application, should be combined with those concerning their viability after drying and also with those concerning the formulation of the final probiotic product to be offered to the consumer [23].
Both high and low-temperature adaptation is equally important during the biomass production based on the industrial platform design. If downstream platform includes a freeze-drying process step, cell adaptation to cold stress is crucial. On the other hand, adaptation of cells to high-temperature stress is important if the downstream process includes spray drying process, which is cost-effective. It has been reported that cells’ exposure to cold stress increases cell viability between 2 log colony forming units (CFU/mL) and 5 log units for Lactiplantibacillus (L.) plantarum and Lacticaseibacillus (L.) paracasei, respectively [24]. On the other hand, exposure to sublethal temperature during exponential growth phase for short period range between 10 and 90 min can enhance cell viability in some Lactobacillus between 10 and 1000-fold depending on the type of strains and fermentation protocol [25, 26]. It has been also reported that high-temperature adaptation during cultivation is associated with the change in cell membrane fatty acid composition [27].
Dehydration techniques such as spray drying, freeze-drying, and vacuum drying are used to produce powdered probiotics containing high cell densities. The technological processing steps can be very tempting for probiotics and cause the limitation of their health benefits. The effectiveness of probiotics is assessed by having at least 107 CFU of beneficial microbes per gram, by plate count enumeration, at the end of their shelf life as it is proposed by the International Dairy Federation (IDF). Probiotics before being included in a food are grown in large numbers by industry and then recovered, concentrated, stabilized, transported, and stored before their final use. Microbial cells are usually preserved and offered in the form of frozen cells, lyophilized cells, or dried cultures [8, 28] in many fermented foods and supplements [29].
Through freezing and thawing of highly concentrated cultures, it is possible to induce cell injury and subsequent loss of viability [30, 31]. Heat is the key contributor in spray drying, affecting a significant number of cell components, as it is the main stress factor [29]. In spray drying, the structure of the viable probiotic cells is affected, specifically the cell membrane and the envelope, causing a decrease in their viability [8] while their metabolic activity is also reduced [32], as a result of the extreme temperature stress [28]. The prevention or limitation of such damages can be achieved by the combination of other techniques, like microencapsulation [33]. Extreme temperatures also from freeze-drying (lyophilization), freezing, and thawing; dehydration from freezing, drying, and/or encapsulation; and osmotic stress upon drying or thawing might affect the probiotic cells in the step of their preparation [8]. Through drying, the cells are quiescent, metabolically inactive, and kept that way until consumption [34]. Dehydration stresses the cell membrane leading to cell death. When water is removed, there is an increase in the ratio between cell surface area and cell volume leading to membrane deformation [29]. During lyophilization, extracellular ice crystals form resulting in an increase in the concentration of medium solutes, causing osmotic stress [34, 35]. Although it is not entirely clear what happens to the inactivation mechanisms in spray drying, modifications in lipid membrane structure and protein denaturation are likely caused by high-temperature exposure, with ribosomal damage being the primary cause of cell death [29].
Fluidized bed drying also causes losses in cell viability due to osmotic stress, excessive dehydration, and oxidative stress [13]. In fluidized bed drying, the probiotic cells are minimally threatened at the typical temperatures used in this technique, up to a moisture level of 15%, while they are more threatened with a decrease in the water activity (aw) of the dry material [34].
Aryaee et al. [36] studied the survival of L. plantarum mixed with blend of fruit juice powder spray and freeze dried. Probiotic cells showed greater viability by freeze-drying compared to spray-drying, which may be attributed to harsher conditions in spray-drying compared to freeze-drying. High survivability in both freeze-dried and spray-dried powders was also demonstrated. The high survivability was also attributed to the use of protective agents and especially the use of malt extract [36]. Malt extracts were also used to increase the viability of L. plantarum in high acidic fruit beverages [37]. Similar results were obtained by Rishabh et al. [32] in which survivability of probiotic strains isolated from the Gundruk (a traditional Indian fermented vegetable food product) was studied among others. The lyophilized carrot juice probiotic powder showed higher viability than the spray-dried form as it had good storage stability reaching 1 month (6–7 log CFU/g).
Food Matrix
The constituents in food, viz., sugars, salts, aroma compounds, natural/artificial flavoring, and coloring agents being the main ingredients in food matrix, could be preservative, impartial, or harmful to probiotic stability [38]. Hereafter, the rapport of probiotics with these distinct food ingredients has a main role in their existence. Naturally, for fermented and non-fermented products, and throughout their storage, these elements could radically touch the probiotic viability and their growth [39, 40]. For instance, higher NaNO2 levels, frequently employed in meat system preservation, provoke a challenge to probiotics in fermentation [41]. On the other hand, several growth agents like glucose, minerals, vitamins, whey protein hydrolysates, casein, and yeast extract, added in dairy products, could enhance the growth rate of Bifidobacteria and Lactobacilli [42]. Practically, in the course of storage, these compounds possess helpful properties on the probiotic survival [43, 44]. Whey protein, hydrolysate acid of casein and tryptone, could stimulate the growth of the probiotic strains (L. acidophilus and Bifidobacteria) by allowing cell nutrition [45, 46]. This fact can be explained by the dropping of the redox potential of the medium and the elevation of the buffering capacity of the medium, resulting in pH decrease [47]. Alternatively, during milk fermentations steps, probiotic (L. acidophilus La-5 and L. rhamnosus Lr-35) growth was reduced; nonetheless, their survival was developed after storage [48]. In this vein, it was concluded that disaccharides could stabilize the cell membrane [49]. For instance, sorbitol can avoid the damage of the cellular membrane stabilizing protein functionality [50,51,52]. On the other hand, some prebiotics as well as oligosaccharides have an affirmative effect on Bifidobacteria viability in food products during storage [53, 54]. Elevated fat content, anaerobic conditions, and buffering potential of the cheese matrix could keep the probiotic cells in the final product throughout intestinal transition [55]. By virtue of the higher values, milk buffering capacity may result in advanced viability of probiotics in dairy fermented products [56, 57]. Additionally, food dry matter could absorb H ions, leading to the organic acid production [58]. Interestingly, delivery of viable probiotic Lactobacilli and Enterococci to the gastrointestinal tract exhibited an additional protecting consequence in cheddar cheese as a food carrier compared to yogurt [59].
An additional factor manipulating the stability of probiotic shelf-life stability was the moisture content of probiotic products [60]. In this line, bacterial survival was conducted by the storage in the existence of both O2 and moisture [61]. Water amounts disturb (i) the viability after drying and its rate of loss during subsequent storage [62]. The optimum moisture content for storage of freeze-dried L. salivarius subsp. salivarius ranged between 2.8 and 5.6%. Increasing the relative humidity (RH) of the environment at which the samples were stored caused an increase in water mobility and in the rate of loss in viability. It should be noted that aw = 0.7 led to a decrease of a 10 log10 cycle of L. rhamnosus GG within 14 days of storage [63, 64].
The packaging features could impact on the probiotic viability. Generally, thickness of the packaging materials, gas, light permeability of polymer, and techniques like active/intelligent packaging systems may have an impact on probiotic viability [57]. In addition, parameters as T and relative humidity could touch the gas permeability of the packaging material and thus disturb the probiotic viability [65]. The incorporation of Bifidobacterium longum NCTC11818 in buffalo curd could establish a probiotic product, and the probiotic strain could survive 3 days in clay pots at 29 °C [49]. Additionally, freezing reduces post-fermentation acidification and extends the strain viability. Interestingly, compared to non-inoculated curd, probiotic buffalo had higher sensory scores. Cruz et al. [66] assessed the probiotic stability in yogurts fortified with glucose oxidase and enveloped in diverse plastic systems with different O2 permeability transfer rates ranging from 0.09 to 0.75 mL O2/day. At lower O2 permeability rates, tested polymer exposed a higher concentration of the probiotic bacteria in yogurts during refrigerated storage. Moreover, inoculated samples displayed a higher extent of post-acidification and organic acid production, since awfully low O2 permeability of glass packages contributed to survival of probiotic cultures.
Storage
After probiotic incorporation on food matrix, certain processes were needed for the stabilization, protection, and product maintenance until being consumed.
Heat Stress
Heat handling, frequently achieved throughout food manufacturing, requires high T that are unfavorable to the majority of microorganisms. Consequently, after subsequent processing, it is desirable to include proper probiotic(s) [67]. At elevated temperatures, between 45 and 80 °C, some probiotic LAB can live for hours. For instance, L. fermentum KGPMF28 and KGPMF2 L. fermentum KGPMF28 are able to grow at 45 °C/24 h [68]. At 55 °C–15 min (heat shock) and 45 °C–30 min (thermal adaptation), L. plantarum strains (Lp 813 and Lp 998) displayed comparable behavior against thermal, osmotic, and oxidative stress factors [69]. Selected thermal adaptation improved the thermal resistance of both strains by 2 log orders. The relevance of cell technological resistance was demonstrated by these authors when choosing possible “probiotic” cultures. Similarly, Haddaji et al. (2015) [70] found that L. casei cells persisted cultivation at 65 °C, which proves that bacteria are talented to withstand such adverse environments.
Physiologically, high T could develop the membrane fluidity and, consequently, interrupt the cell activity [71]. To prevent the degradation and denaturation of probiotic LAB, Chen et al. (2017) [72] confirmed that L. kefiranofaciens M1 possesses a diversity of adaptation mechanisms, comprising the production increase on specific evolutionarily conserved proteins. These proteins include HSPs and enzymes, viz., PtsI, DnaK ProS, GroEL, and GroES, that take a leading role in endorsing proper folding and a consequent translocation of nascent polypeptides [73]. In addition, probiotics that grow under heat stress possess saturated and straight-chain fatty acids, which offer the correct fluidity required for membrane function [74]. The expression of DNA-binding proteins stands as an alternative approach to biomolecules keep akin to DNA [75]. The efficiency of these preserved proteins in cells from heat stress can differ from species to species. For example, a heat tolerance study within the Lactobacillus genus displayed that genetic variation/environmental factors, such as culture media, NaCl concentration, aw, and pH, expressively could impact on strain resistance to heat stress [76].
Cold Stress
Some probiotics could grow at T < 15 °C and adapt in numerous cold environments. In this line, the probiotic viability during cold temperature storage of fermented food before consumption is a decisive factor for their functional characteristics [77]. A rapid temperature decrease can convince physiological stresses via reducing membrane fluidity and DNA altering supercoiling and RNA, which may disturb replication, transcription, and protein production [78]. In cold T, the components and enzymes of probiotics developed a rigid, provoking toxicity [79]. Additionally, at very low temperatures, ice crystals formed probiotic cell membranes, injuring or killing the cells [80]. Furthermore, cold stress conditions should also kill cells after freezing. As an example, B. subtilis growing is detained upon a T deceleration, causing the hindering probiotic protein synthesis [81]. LAB probiotics could overcome these damaging impacts and be functional at low T by generating antifreeze and CSPs, by controlling the expression of cold-induced genes (as antiterminators), which improve detrimental effects related to cold environments [82]. On the other hand, probiotic LABs are familiar to produce cold-adapted enzymes that sustain activity at freezing T° and provision both transcription/translation [83]. Some probiotic strains are also qualified by antifreeze proteins joined to ice crystals and stop them from intense cells [84].
Stress Conditions Faced by Probiotic Microorganisms During Gastrointestinal Passage
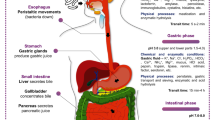
After ingestion, the survival of probiotics is greatly compromised by the unique environment of the human gastrointestinal tract (GIT), where they are exposed to a plethora of harsh physicochemical conditions [85]. Salivary enzymes, the low pH of the stomach, and the presence of bile in the intestine are crucial barriers that probiotic bacteria have to overcome to exert their beneficial action (Fig. 1).
Orally administered probiotics are initially exposed to saliva, a complex fluid containing water, mucus, electrolytes, and enzymes, with a pH in the range between 6.2 and 7.4, among which lysozyme stands out for its antimicrobial properties [86, 87]. In addition, saliva contains immunological components and receptor proteins that can block microbial surface adhesins and modulate colonization in oral tissues. However, available studies suggest the zero impact of saliva components on the viability and colonization capacity of probiotics, as the exposure time is transient [88].
Stomach Environment
The next critical stage in the probiotics’ long journey through the GIT is the stomach, where the most detrimental condition is created by acidic gastric fluid (Fig. 1). Between 1.5 and 2.0 L of gastric juice is produced per day, consisting of water, hydrochloric acid, electrolytes, mucus, and digestive enzymes such as lipase and pepsinogen, among others. During digestion, the secretions of the parietal cells mix with the food bolus so that the pH of the stomach oscillates between 0.9 and 3.0, during a transit time of 5 min to 2 h [11]. An antimicrobial effect is exerted by the unique composition and low pH of gastric secretions, in which allochthonous microorganisms, such as probiotics, can be rapidly destroyed.
An acidic environment can cause a reduction in bacterial intracellular pH (pHi), alter the anion pool, affect the integrity of DNA bases, and cause denaturation of essential enzymes, including a decrease in the activity of glycolytic enzymes and F1F0-ATPase proton pumps [85, 88].
As it is known in bacteria, F1F0-ATPase generates ATP when extracellular protons cross the cell membrane into the cytoplasm across a pH gradient [89]. However, in an acidic environment, the accumulation of H+ causes the pumps to consume ATP, depleting the available energy and leading to cell death [85].
In addition, low pH can damage the composition, integrity, and functionality of plasma membranes [85, 90]. Indeed, exposure to acid can induce several modifications in cell membrane composition of proteins and lipids, alter the diffusion of molecules, and modify peptidoglycan components. Considering that cell membranes provide a constant intracellular environment to maintain cellular activities and that they also participate in the stress response of cells, these modifications could lead inexorably to death in non-adapted cells [85]. For example, potential probiotic L. plantarum UBLP40 survival was significantly reduced (p ≤ 0.0001) to zero within 1 h at pH 1.0 compared to the control at pH 7.3, while at pH 2.0 and 3.0, 73–95% and 93–100% survival was detected, respectively, depending on exposure time [91]. Furthermore, Sesín et al. (2023) [92] reported that 15 out of 17 LAB isolated from goat cheese showed a survival rate higher than 75% after 3 h of exposure at pH 2.5. Finally, when different strains of Lactococcus lactis and Leuconostoc lactis were incubated for 12 h in MRS at pH 2.0, 3.0, and 4.0, a viability of 24–27, 8–9%, and 6–7%, respectively, was recorded. On the contrary, exposure of cells to simulated gastric juice at pH 3.0 for 3 h showed survival of 100% [93].
Intestinal Stay
Once in the gut, the main factors that regulate the establishment and permanent existence of bacteria include the disruptive activity of digestive enzymes, sudden pH changes, detergent properties of bile acids (BAs), and molecules produced by the host’s immune system [94, 95]. Indeed, when passing from the stomach to the intestine, probiotics are first confronted with a sharp rise in pH from 2.0 to 6.0 mainly due to the presence of bicarbonate (Fig. 1). This abrupt change can damage membranes and alter the structure of proteins and DNA, among others, thus compromising microbial viability [11]. In addition, the cells are exposed to the action of pancreatic secretions, mainly composed of digestive enzymes such as proteases (trypsin and chymotrypsin), amylases, and lipases [96]. The digestive enzymes have been shown to reduce the adhesion to intestinal mucus of probiotic strains [97]; however, the effect of pancreatic secretions on microbial viability has not been studied in depth.
Bile secretions are produced and secreted by the liver, stored in the gallbladder, and secreted into the duodenum. It consists of an alkaline solution with a pH between 7 and 8 whose main components are BAs, biological detergents involved in the emulsification and absorption of dietary lipids. The concentration of BAs ranges from 12 mM in the duodenum after a high lipid intake to 2 mM in the ileum due to active reabsorption [98]. The primary BAs, chenodeoxycholic acid and cholic acid, are synthesized in the liver from cholesterol, in a multienzyme process [94]. Before leaving the liver, its steroid nucleus is conjugated with taurine or glycine (predominantly in humans) to increase its solubility. Conjugated BAs are accumulated in the gallbladder and released to the duodenum after food intake, contributing to the solubilization of ingested lipids. Then, they are reabsorbed and returned to the liver via enterohepatic circulation process [99].
Non-reabsorbed BAs are strongly modified by intestinal microbes and primary BAs are converted to over 20 different secondary metabolites. Indeed, composition of BAs is strongly affected by gut bacteria, and reciprocally, they are the major regulators of shape and composition of gut microbiota [100]. The main reaction of BA metabolism is the hydrolysis of the amide bond to release the free bile acid plus the amino acid, reaction performed by microbial enzymes collectively called bile salt hydrolase (BSH) [96].
Bacterial membranes are the main target of the antimicrobial action of BAs due to their amphipathic character that confers detersive properties. In general, LAB are thought to accumulate in the lipid bilayer and then penetrate into the cell interior. In this sense, antimicrobial activity is related to the chemical structure and hydrophobic properties of BAs as they can diffuse and accumulate more easily in the lipid bilayer [101].
Electron microscopy studies revealed that exposure to BAs resulted in alterations in the membranes which showed folding and budding [102], while the cytoplasm decreased and the membranes became thin and rough [103]. Moreover, BA incorporation into the phospholipid bilayer can interfere with phospholipid molecules’ normal arrangement thus leading to a loss of membrane integrity in many probiotic bacteria.
In bacterial cells, the proton driving force, composed by the transmembrane electrical potential (ΔΨ) and the pH gradient (ΔpH), supplies the electrochemical energy needed for cell growth and ATP synthesis, pH homeostasis, membrane transport, motility, stress resistance, cell division, electrical communication, and environmental sensing [104]. Consequently, disruption of the proton motive force has severe consequences for the cell, even leading to cell death. Dissipation of ΔΨ and ΔpH accompanied by intracellular acidification and intracellular ATP depletion and leakage of essential ions and small molecules was reported in probiotic strains exposed to conjugated and free BAs [98, 105,106,107]. Moreover, oxidative damage to DNA and RNA and protein misfolding could be induced by BAs [108, 109]. In this regard, Bustos et al. [109] reported that deoxycholic acid induces greater changes in the secondary structure of a model protein than taurodeoxycholic acid, modifying surface charges and inducing the formation of protein aggregates, resulting in a loss of activity. In addition, deoxycholic acid interacts in the active site of the enzyme while taurodeoxycholic acid does not, probably due to the presence of taurine as shown by molecular docking studies.
Understanding the Adaptive Mechanisms of Probiotic Bacteria to Gastrointestinal Stress
Microbial responses to stress, including those of the GIT, are multifactorial phenomena; however, they can be broadly classified into (i) innate or intrinsic mechanisms and (ii) adaptive mechanisms. The former includes structures and metabolic pathways naturally present in the microbial cell that allow tolerance to the stressor. Adaptive responses involve genotypic and phenotypic modifications, which may be associated with mutations that arise following the exposure of cells to stress and allow microorganisms to survive in its presence [11]. Probiotic microorganisms have developed common coping strategies to deal with both acid and bile stress, as well as other types of environmental stress, as mentioned above. The most common defense mechanisms include regulation of energy production by modulation of different metabolic pathways, modification of cell envelope and membrane lipid composition, overexpression of chaperones, stress proteins, and macromolecule repair enzymes, among others (Fig. 1, Table 1).
Resistance strategies developed by probiotic bacteria to GIT stresses can be studied using physiological, biochemical, and genetic analyses, while omics approaches have recently proved valuable in discovering new biomarkers of stress resistance. Indeed, the advent of omics technologies has enabled a deeper and more holistic understanding of probiotic biology and response mechanisms, which has prompted the search for new ways to screen for the best candidates.
Innate and Adaptive Mechanisms Identified in Probiotic Strains in Response to Acid Stress
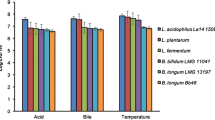
Since stomach acidity represents one of the main survival challenges for probiotic microbes, sophisticated mechanisms at the physiological and molecular level have been devised to survive and adapt to acid stress. In addition, the mechanisms of acid tolerance in different probiotic microorganisms have been investigated by many different approaches. Some of the responses to acid stress in various beneficial bacteria are listed in Table 1.
Maintaining pH homeostasis, i.e., pH regulation inside and outside the cell, is a strategy that some microorganisms have developed to resist acid stress [133, 134]. As a strategy, certain yeasts and bacteria, when exposed to a changing extracellular pH, can maintain a near-neutral pHi, quite stable, and generate unfixed proton gradients [85]. However, maintaining a stable pHi requires significant energy consumption, with the immediate result the restriction of the growth and metabolism of microbes. In contrast, most acid-tolerant microbes, such as LAB, have developed a different strategy for survival, by maintaining a constant pH gradient rather than a constant pHin [89]. Thus, the pHi of these microbes decreases as the pHext decreases but remains at a level above the pHex [135]. Using this strategy, it is advantageous for LAB because proton translocation consumes energy, and these fermenting bacteria obtain significantly less energy from sugar metabolism than aerobic bacteria. A positive ∆pH is critical for many cellular bacterial processes, such as cell growth, energy uptake by ATP synthesis, DNA replication, transcription, and translation, among others. However, as acid continues to enter the cell, a critical concentration is reached after which the pHi decreases sharply; the ∆pH collapses or approaches zero, disrupting vital cellular processes and resulting in a loss of cell viability [98]. Therefore, pH homeostasis maintenance is required for survival of microbes in acidic environments.
In this sense, microbial cells can maintain pH homeostasis by different strategies, including (i) modulating cell membrane permeability and modifying channel size to restrict proton entry, (ii) removing excess protons from the cytoplasm via the proton pump, (iii) diverting proton entry by formation of chemiosmotic gradients via potassium ATPases, and (iv) maintaining membrane fluidity, characteristics that are determined by the acyl fatty acid chain composition and the head group [85]. In a study of wild-type L. casei Zhang and its acid-resistant mutant, it was observed that in response to acid stress, the cell membrane fluidity decreased and reduction of acid damage was achieved by a change in membrane’s fatty acid composition. Compared to the wild-type strain, the mutant had higher proportions of unsaturated fatty acids and a longer average chain length [111].
Using omics tools, key mechanisms of adaptation to acid stress were identified in probiotic strains. In a comprehensive work by Wei et al. [120], the differential gene expression of the probiotic strain Bifidobacterium longum JDM301 and its stress-adapted derivative strain was evaluated. The resistant strain was obtained by 150 batch subcultures. Both strains were grown at an initial pH of 6.5 or pH 3.5, and high-throughput RNA sequencing was carried out to analyze changes in the gene expression profile. Notably, genes encoding ammonium transporters and the enzyme cystathionine gamma-synthetase were upregulated under acid stress in both strains. Cystathionine gamma-synthetase is responsible for the synthesis of cystathionine, which can then be converted to ammonium. Similar results were reported by Sánchez et al. [121] in an acid-resistant mutant of Bifidobacterium longum, demonstrating the importance of ammonium in the neutralization of excess protons. In addition, some genes involved in peptidoglycan synthesis and fatty acid metabolism were upregulated in Bifidobacterium longum JDM301 under both normal and acid stress conditions [120]. These findings suggest that cell envelope and membrane repair are important in both adaptation and acid resistance of the strain since they are the first targets of action of several stresses. Similarly, Jin et al. [122] reported overexpression of proteins involved in peptidoglycan synthesis, accompanied by an increase in their production, in Bifidobacterium longum BBMN68 pre-stressed by exposure to a sublethal pH and subsequently subjected to pH 3.5. In addition, pre-stressing increased the abundance of proteins involved in energy production, amino acid metabolism, and ATP and NH3 content, thiols, and H+-ATPase activity relative to uninduced cells.
Other proteins, normally affected in acid-exposed probiotic cells, belong to the following functional categories: translation and transcription, macromolecule protection and repair, stress proteins such as chaperones, energy production and conversion, carbohydrate transport and metabolism, and amino acid transport and metabolism, in particular, the transport and deamination of branched-chain amino acids, which has been postulated as a mechanism for maintaining bacterial internal pH [120, 122, 124]. Surprisingly, repression of genes involved in cell division, in particular the tubulin analogue FtsZ, was observed, a finding that has also been described in bacteria under other types of environmental stress [120].
Innate and Adaptive Mechanisms Identified in Probiotic Strains in Response to Bile and Bile Acid Stress
Given the complicated nature of bile stress, different defense mechanisms should be deployed by microorganisms in order to overcome its presence. BAs are the major constituents of bile and are mainly responsible for its antimicrobial effect. Moreover, tolerance to BAs is strain-dependent and shows extreme variability even within the same genus or species. As already mentioned, omics technologies allowed the characterization of the responses of probiotic bacteria to different types of stress, including the presence of bile and bile acids. Furthermore, using omics approaches, specific bacterial biomarkers were proposed to find the best strains with probiotic potential. Some of the responses to bile stress in various beneficial bacteria are listed in Table 1.
Resistance of the presence of bile in probiotic bacteria by the intrinsic resistance mechanisms is related to changes in the architecture and composition of the cell membrane and the presence of efflux pumps and by the ability of the cells to maintain intracellular homeostasis. In addition, BSH enzyme has been recommended to participate in a potential detoxification mechanism for the resistance of the presence of BAs [94, 113, 136]. Accordingly, omics studies show that the main genes and proteins expressed in response to bile in probiotic bacteria are related to fatty acid biosynthesis, the metabolism of carbohydrates, amino acids and nitrogenous bases, bile salt transporters, and stress response proteins [113, 115, 117, 123, 128].
Exposure to bile and bile acids has been reported to induce changes in membrane lipid and fatty acid composition in probiotic Bifidobacteria and Lactobacilli, which may contribute to their stress tolerance [112, 137]. To evaluate the adaptation mechanisms of a probiotic strain of L. gasseri JCM1131T, a BA-resistant strain was obtained by Kato et al. [113] through pre-exposure to sublethal concentrations of cholic acid. Adaptation resulted in reduced cell membrane damage and increased abundance of long-chain sugar glycolipids, as well as a doubling of cardiolipin content. In addition, cardiolipin reduced phospholipid vesicle solubilization after cholic acid exposure, suggesting that it plays a significant role in bile acid resistance and cell membrane maintenance. Similar finding were recently reported by Shimizu et al. [113] in L. paracasei strain Shirota. In this line, it was reported that the addition of soy lecithin, as a source of phospholipids, is able to modulate the surface properties and bile resistance of L. plantarum strains, confirming the important role of membrane composition in bacterial tolerance to bile [138]. In addition, a recent study showed that the addition of a squalene synthase inhibitor increases the susceptibility of Akkermansia muciniphila to BAs by changes in membrane structure. Akkermansia muciniphila is an intestinal commensal bacterium that has recently attracted the attention of researchers due to its probiotic effects related to amelioration of obesity and metabolic disorders and modulation of the host immune response [128]. In addition, transcriptome analysis of Akkermansia muciniphila DSM 22959 showed the upregulation of proteins associated with hopanoid production in the presence of BAs. These results indicate a probable BA tolerance in Akkermansia by hopanoid production, associated with membrane permeability [128].
On the other hand, the cell wall is also crucial for the maintenance of cell homeostasis under conditions of environmental stress. Peptidoglycan synthesis is a complex phenomenon, controlled by numerous proteins encoded by the bacterial genome. In this regard, Ali et al. [116] detected that 35 proteins related to cell wall biosynthesis were upregulated in Lactobacillus fermentum NCDC 605 in response to bile. Furthermore, research showed that cell wall synthesizing enzyme in Gram-positive and Gram-negative bacteria, UDP-N-acetylmuramate-L-alanine ligase, was upregulated and overexpressed in P pentosaceus M41. This enzyme is related to the strain’s tolerance to different types of stresses, such as acid and thermal as well as BAs [123]. Finally, enzymes involved in cell surface charge modification and a hemolysin-like cell envelope protein were overproduced in the presence of bile in Lactobacillus salivarius Ren, probably to prevent bile adsorption [117].
The upregulation of efflux transporters was reported in many probiotic strains to reduce the intracellular accumulation of BAs [117]. In this sense, transcriptome and proteomic analysis of Akkermansia muciniphila, L. reuteri, L. salivarius, and Lactobacillus mucosae showed that transporter gene clusters were upregulated in the presence of bile and BAs [117, 118, 128, 129].
In response to stress, bacterial cells increase carbohydrate metabolism because maintaining fundamental cellular processes under these conditions requires high energy production. In this regard, Ali et al. [116] identified 53 proteins involved in various carbohydrate metabolic pathways, including glycolysis, pentose phosphate pathway, galactose synthesis and glutamine pathways, while exoproteomes analysis of L. johnsonii and L mucosae revealed overexpression of proteins involved in glycolysis, such as L-lactate dehydrogenase, fructose-bisphosphate aldolase, glucose-6-phosphate isomerase, triose phosphate isomerase, phosphoglycerate kinase, phosphoglycerate kinase, and phosphofructokinase [118, 119]. These pathways aim to provide ATP to meet the energy demands of cells under stress conditions. Moreover, Wang et al. [117] employed transcriptomic and proteomic analysis to describe adaptation mechanism of L. salivarius Ren exposed to 0.75 g/L ox-bile. Surprisingly, the maltose utilization pathway, whose metabolism produces more than twice as much ATP as glucose metabolism, was overexpressed in the presence of bile. It was previously reported on the preference of maltose over glucose compared to the parental strain for a bile-resistant derivative of Bifidobacterium animalis, probably due to non-availability of glucose in the distal colon [130].
Proteins and genes involved in transcription and translation, as well as in the general stress response, are increased in the presence of bile, as this stress affects several cellular systems. These include a large number of highly conserved proteins in bacteria that are often involved in the maturation of new proteins, refolding or degradation of denatured proteins, and DNA repair [139]. The expression of these stress-response proteins was also induced by other stresses including acid, heat, cold, and osmotic stress, suggesting that this system is a general adaptive mechanism in probiotic bacteria [90, 139]. Chaperones such as several heat shock proteins (Hsp), GroEL, GroES, DnaJ, and DnaK as well as elongation factor were induced to counteract with bile and BAs in L. reuteri CRL 1098 [115], L. fermentum NCDC 605 [116], L. salivarius Ren [117], L. johnsonii PF01 [118], L. mucosae LM1 [119], among others.
Finally, some evidence suggests that BSH activity might affect bile tolerance in some Gram-positive bacteria as part of a cell detoxification strategy [136, 140]. As stated above, the main reaction in BA metabolism is performed by BSH enzymes. BSH activity has been described in bacterial genera of intestinal origin, including former genus Lactobacillus, Bifidobacterium, Clostridium, Enterococcus, Listeria monocytogenes, and Brucella abortus, among others but not exclusively. The potential role of BSH activity in detoxification has been discussed, as free BA are less soluble, precipitate at intestinal pH, and leave the GIT with the feces, decreasing their interaction with gut bacteria [136]. An early metagenomic study [141] links resistance to conjugated BAs of intestinal microorganisms with the presence of BSH activity. An interesting work by O’Flaherty et al. [142] investigated the presence of bsh genes in 170 lactobacillus genomes. The results revealed that species harboring bsh genes are mainly associated with vertebrate-adapted niches, suggesting a selective pressure on lactobacilli to evolve and adapt to specific environments.
Changes in BSH expression at the transcriptomic and proteomic level in response to bile have been reported in previous studies. A proteomic study revealed the repression of the expression of the BSH enzyme by a bile-resistant strain of L. plantarum, while sensitive strains showed no change [143]. Moreover, a bile-adapted strain of Bifidobacterium animalis showed overexpression of the BSH enzyme compared to its wild-type counterpart. The same thing was observed in Bifidobacterium longum exposed to an intestinal environment [121, 132, 133]. Finally, neither Enterococcus faecalis V583 nor L. reuteri ATCC 23272 modified BSH enzyme expression at proteomic level in the presence of bile [144, 145].
Improvement of Multiple Stress Tolerance of Lactic Acid Bacteria
The challenge of improving probiotics survival rate during the different steps of the bioprocess that includes biomass production, dehydration (freezing, freeze-drying, spray drying), and long-term storage of the products is included in probiotic production. A probiotic must be able to tolerate exposure to a number of different stressors during its lifetime. The stress tolerance of Lactobacillus species can be strain-specific, so stress tolerance is a crucial factor in selecting strains for use as probiotics or in other industrial applications. Researchers and manufacturers often assess these properties when characterizing and selecting specific Lactobacillus strains for various purposes. By reason of the various stressors that can affect the viability of probiotics during production and storage was require a number of technological strategies to help minimize the effect of stressors.
Strategies in the Probiotic Bioreactor Production Stage
The physiological state of the pre-process culture is key to the intrinsic tolerance of the cells to stress conditions [146,147,148]. Bacterial cells can be prepared to cope with different adverse environments by modifications in fermentation conditions. These include culture medium composition, temperature, pH, culture age, and atmosphere (gas injection). Lactic acid is produced by LAB due to their type of metabolism, leading to a decrease in the pH of the culture. Although LAB can grow in a wide pH range (3.5–6.5), the cultures in stationary phase are subjected to acid stress which limits their growth and may affect their resistance to subsequent stress factors. Thus, avoidance of cell injury and improvement of biomass yield are achieved by pH-controlled fermentations [149]. Contrary, Ai et al. [150] reported on L. bulgaricus Q7 where the average growth rate and the final biomass were higher under free pH conditions. At this point, it is necessary to differentiate biomass yield with the possibility of generating a technologically robust biomass with a resistance to multiple stress situations. Inevitably, the biomass obtained in bioreactors must subsequently withstand multiple technological processes and the metabolic condition of the biomass will determine its survival. Recently, the effect of culture parameters (pH, growth phase) on cell viability and heat tolerance of probiotic L. rhamnosus CRL1505 was evaluated [149]. These research works reported that running fermentations at pH 5.5 and harvesting the cells at the exponential phase are the best conditions for obtaining a high live biomass yield capable of overcoming heat stress.
The production of probiotic cultures on an industrial level only considers the highest biomass without taking into account their condition. Thus, they are generally harvested in the stationary phase, in which there is a high percentage of damaged cells, often unable to survive subsequent stress factors. If we consider the strategy of pH-controlled cultivation to minimize this damage, the literature search indicates that the results are strain-dependent. At pH 6.0, some probiotic cultures showed a low survival to heat, oxidative, and osmotic stress [149, 151].
Depending on the composition of the culture medium, during growth, some microorganisms accumulate compatible solutes to maintain osmotic equilibrium with the extracellular environment. Compatible solutes, including betaine, carnitine, and proline, refer to the accumulation of protective compounds. LAB do not synthesize compatible solutes and therefore depend on the environment to take them up [152]. Compatible solutes can facilitate the stabilization of proteins by microbial cultures and the cell membrane during osmotic stress conditions caused by low water activity during the drying process [153, 154]. In this regard, Huang et al. [148] proposed that cytoplasmic accumulation of inorganic polyphosphate (polyP) in Propionibacterium freudenreichii is osmotically induced and utilized as energy storage molecules and compatible solutes. The accumulation of polyP in this microorganism led to increased multistress resistance and, particularly, to increased survival after spray drying [153, 155]. Extensive studies of the synthesis of polyP in microorganisms have been reported due to its use in bacterial physiology. The synthesis of polyP by some probiotics depends on the concentration of phosphate in the culture medium and the growth phase, so the formulation of a suitable medium and the harvesting of biomass at the appropriate stage may be an appropriate strategy to favor the survival of the probiotic to the drying processes.
The synthesis of chaperone proteins is one of the protective strategies employed by probiotics to cope with heat stress [156]. Stabilization of protein structure and function can be achieved by this mechanism, thus contributing to maintenance of optimal metabolic performance under various stress conditions [157]. At this point, mutants with an overexpression of chaperones can be generated. Corcoran et al. [158] reported an overproduction of GroESL by the probiotic strain Lactobacillus paracasei. This strain showed (higher survival to spray and freeze-drying) compared to the unmodified wild-type strain.
Bacteria have evolved mechanisms (uptake and synthesis systems) for the accumulation of compatible solutes [157]. Three distinct uptake systems (BetL, Gbu, and OpuC) and a compatible solute synthesis system (ProBA) of the food pathogen Listeria monocytogenes have been discussed in probiotics [159]. The transcriptional control of the nisin-inducible promoter PnisA to assess the role of BetL (and thus betaine accumulation) in contributing to the survival of Lactobacillus salivarius UCC118 in a variety of stress situations might be responsible, according to some authors, for the betL gene (encoding the BetL betaine uptake system) [160, 161]. L. salivarius showed a significant increase in betaine accumulation and increased resistance to stress factors compared to the wild type.
Oxygen is an important factor for probiotic bacteria, as it affects both positively and negatively their growth. Most of the growth of probiotic lactobacillus species depends on the presence of electron acceptors as they are considered oxygen-tolerant anaerobes because they do not have a complete electron transport chain [162]. The presence of oxygen incorporated into the bioreactor by agitation can induce the production of reactive oxygen species (ROS) that lead to damage of cellular components, such as proteins, lipids, and nucleic acids [163]. Thus, one strategy is to add electron-accepting molecules [164, 165] to the substrate for aerobic culture and minimize agitation during production. By keeping oxygen at a sufficient minimum level, maximization of biomass yield and avoidance of oxidative stress can be achieved [164, 166]. In the most recent study of Rao et al., a significantly higher survival rate of Limosilactobacillus (L.) reuteri DSM 17938 was observed in the lyophilized product for cells cultured both in the presence and absence of oxygen (61.8% ± 2.4% vs. 11.5% ± 4.3) [12]. On the contrary, during drying, oxygen-sensitive bacteria, e.g., Bifidobacteria, due to the lack of oxygen in the drying environment, may result in a reduction in oxidative stress [13].
Improvement of Survival During the Dehydration Process
Freeze-drying is an efficient and widely used technique for preserving probiotics. By offering increased stability and viability, freeze-drying allows probiotics to maintain their biological activity and health benefits during storage. Freezing and osmotic stress reduce bacterial enzyme activity [167, 168]. The freezing stage prior to water sublimation induces crystal formation, which stiffens LAB cell membranes and reduces membrane fluidity [92]. To overcome the adverse situation, LAB have implemented a number of adaptive mechanisms. Derzelle et al. [169] suggested that cold stress is alleviated by upregulation of cold shock proteins (CSPs) (CspL, CspP, and CspC) [170]. In addition, it is possible to increase cryotolerance by accumulation of compatible solutes. Overexpression of BteL, the betaine uptake system in L. salivarius, led to the accumulation of betaine glycine, which increased resistance during freeze-drying [161]. Survival of L. bulgaricus in freeze-drying was improved if the cells were subjected to osmotic stress [171]. Osmotic stress led to stress adaptation by overexpression of the compatible solute uptake system, leading to its accumulation and thus enhancing freeze-drying tolerance. Osmotic stress adaptation can be induced by the addition of salt and compatible solutes such as trehalose. Trehalose can improve the survival of LAB during freeze-drying [172]. The accumulation of intracellular trehalose caused by osmotic stress leads to improvement of survival during freeze-drying.
In addition, improvement of survival rate of LAB can be implemented by the exogenous addition of suitable protective agents and by adjustment of the freeze-drying process [173]. The literature suggests that the best approach to increase survival is the use of a cryoprotectant [172]. Cryoprotectants such as sucrose, lactose, sorbitol, and skim milk have been proven to be effective in improving the survival of probiotic bacteria during freeze-drying and storage [174]. The synergistic combination of cryoprotectants, namely, 6% sucrose/8% skimmed milk/4% monosodium glutamate, gave a high survival rate of Streptococcus thermophilus by lyophilization of 90.59% [175].
The application of the spray-drying technique to probiotics is reduced because, during drying, the cells encounter different stress conditions that can affect their viability. These conditions include heat, osmotic, and oxidative stress; dehydration; and shear stress, a process during which particles are deformed by the action of a shearing external force during atomization [176, 177]. The heat stress and dehydration are the two main mechanisms leading to inactivation and loss of viability of probiotics [178]. However, there is a big variation between different bacterial genera and species as mentioned before [34], and therefore, monitoring on a case-by-case basis is required. It is important to maintain the probiotic properties of LAB during a bioprocess such as spray drying. The tolerance of bacteria to stress derived from the spray-drying process depends on the intrinsic tolerance of the microorganisms, the conditions of biomass production (fermentation) [152, 179], addition of protective agents, adaptation of the cells to the process parameters, and sublethal stress pre-treatments prior to drying [152]. These strategies affect the viability of probiotics immediately after drying, but also during storage. Figure 2 outlines examples of strategies to increase cellular tolerance to stress and generate protection that contribute to reducing the negative effects of spray drying.
The coating material that protects the cells from upcoming environmental stress is another crucial parameter for probiotic cell viability. Whey protein isolate (WPI) and gum arabic (GA) have been used as spray-dried encapsulation materials because of their ability to form physically strong and stable matrices [3] and also have been combined in order to increase their efficiency [180,181,182]. Tirta et al. reported that Pediococcus (P.) acidilactici cell viability was significantly affected by inlet temperature when whey protein and GA coating materials were used, but not by wall material ratios during a spray drying process. When the inlet temperature was increased to 170 °C, this caused a decrease in the viability of P. acidilactici by 1.36 log cycles, from 8.61 log CFU/g to 7.25 log CFU/g [3].
The drying matrix and the addition of thermoprotectants are another aspect to consider for improvement of the survival of probiotics to spray drying. These compounds show thermoprotective features, such as disaccharides (lactose, sucrose, or trehalose), dextrose, or polyols (mannitol, sorbitol), or act as probiotic growth stimulants (fructo- and galactooligosaccharides) [181,182,183,184,185,186]. An example of heat protectants to drying media (solid support) is sugars, which are frequently added in the production of dried starter cultures. They also contribute in the improvement of the survival rate of the cells during processing and further storage [187]. Oldenhof et al. [188] discussed the use of a mixture of maltodextrin and sucrose, leading to an increased survival in spray-dried lactobacilli. Sugar interacted well with lipids and proteins, and the maltodextrin functioned as an osmotically inactive loading compound leading to the formation of a glassy matrix [188]. Molecular movement is restricted by this glassy matrix and thus retards crystallization and diffusion processes, deteriorating external effects and bacterial metabolism [34]. The retail price for sucrose is approx. equal to US$ 1.45/L, used at 10% (w/v), and affecting the cost of bulk starter production. Correa Deza et al. [149, 189] formulated the thermoprotective additive containing only inorganic salts (MnSO4, MgSO4, KH2PO4, and Na2HPO4), which was successful for improvement of the cell viability of probiotic CRL-1505 strain during spray drying. These results are interesting from a technological point of view considering the price of phosphate salts (about US$ 0.40/L) and the possibility of being used as thermoprotectant during spray drying.
The induced damage can be reduced by the adaptation of LAB in physiological and metabolic aspects [77]. Mild stress adaptations including synthesis of stress protein(s), change in the fatty acid composition of the cell membrane, and transport or synthesis of compatible solutes [190] improve tolerance to multienvironmental stress by resistance of both the particular stress and other unrelated stresses [70].
Setting of Technological Parameters
During industrial application of LAB, bacteria are subjected to various environmental stresses, hence the restriction of the growth and alteration of the physiological and biochemical properties of cells. Among the strategies that could be applied before the LAB is subjected to the dehydration process itself, we can mention protective pre-treatments to create adaptation (changes in membrane, production of stress proteins, accumulation of compatible solutes). If the previously detailed strategies are not efficient enough to achieve adequate survival, technological parameters of the dehydration process can still be adjusted. The adjustment of optimal conditions of freezing time, freezing temperature, pressure, and lyophilization time during lyophilization determines the survival of the probiotic not only at the end of the process but also during storage [117, 191].
The negative effect of the spray drying process, besides being related to intricate characteristics of the probiotic LAB, depends on the process parameters (inlet and outlet air temperature, feed flow rate, drying chamber time, drying chamber design, drying medium temperature) employed during dehydration [180, 183, 192, 193]. The use of high levels of feed flow rates can reduce heat-induced cell damage, however will result in a high water activity powder with a short shelf life [193, 194]. For most heat-sensitive strains, such as L. acidophilus or L. rhamnosus GG, an air outlet temperature between 70 and 80 °C is recommended to minimize cell injury induced by spray drying [181,182,183, 195].
Postbiotics: A Strategy for Maintaining the Health-Promoting Properties of Inactivated Probiotics
Although the traditional probiotic definition presupposes that bacteria must remain alive to produce health-promoting effects, much scientific evidence has shown that formulations containing their cellular byproducts can also promote the desired response. These compounds are called postbiotics and include cell lysates (CLs), enzymes, and cell wall fragments derived from probiotic bacteria and can be an alternative to probiotics, taking into account that strain-specific behavior, antibiotic gene transfer, and the potential of some probiotic strains for infection in immunocompromised individuals could be some of the limitations of probiotics [196, 197]. When ingested in sufficient quantities, the host is provided with multiple biological health benefits [198, 199]. Some of the many postbiotic metabolites include exopolysaccharides, glycoproteins, peptides, proteins, peptidoglycans, linoleic acid, lactic acid, and short-chain fatty acids shown to have significant antioxidant, anti-inflammation, and antibacterial effects [199, 200].
Moreover, the clinical and health benefits of probiotics are not associated with viability due to the non-interdependence of the plausible mechanisms with viability as reported by Barros et al. [198]. Postbiotics possess potential health-promoting properties, and a recent study by Park et al. [199] investigated the effect of CLs of Lactiplantibacillus plantarum (LP CL) and Lacticaseibacillus rhamnosus GG (LR CL) on the inhibition of virus-mediated inflammatory responses in the human intestinal epithelial cell line HT-29 in vitro.
They found that both LP CL and LR CL could inhibit virus-mediated inflammatory responses and confer synergistic inhibitory effects with short-chain fatty acids such as butyrate in human intestinal epithelial cells. Hosseini et al. [200] studied the potential biological activities of postbiotics derived from Saccharomyces cerevisiae (PTCC 5269) (PSC) under in vitro circumstances. A high level of phenolics and flavonoids was detected with strong antibacterial activity against pathogens (creating eubiosis) showing that their health-promoting functions can be extended to medical, biomedical, and food scopes, for design of optimized functional food formulations or/and supplementary medications for prevention and treatment of chronic/acute disorders. Postbiotics improve the efficiency of the innate immune system, decrease the inflammatory responses caused by the presence and activity of pathogenic germs with inflammation-inducing activity and carcinogenic agents (especially those derived from food processing), and bolster the effectiveness of the intestinal barrier [204,205,206].
Conclusions
Given the relationship between the microbiome and several diseases or specific symptoms, research effort should be focused on the optimal intervention of probiotics as a complementary tool for their treatment. Since the results are different between different bacterial genera and species, but also between strains of the same species, their study must be done separately for each. Further research to optimize all the conditions that contribute to dealing with stress to which probiotics are subjected and increasing their bioavailability will bring about new uses for probiotics contributing to issues concerning both the food industry and other similar industries.
Data Availability
No datasets were generated or analyzed during the current study.
Abbreviations
- HSPs:
-
Shock proteins
- PtsI:
-
Phosphoenolpyruvate-protein phosphotransferase
- DnaK:
-
Gene encoding the DnaK chaperone protein
- ProS:
-
Prolyl-tRNA synthetase
- ProRS:
-
Chaperone protein DnaK
- GroEL:
-
Chaperonins
- Cofactors:
-
GroES
- CSPs:
-
Cold-shock proteins
- RH:
-
Relative humidity
- HDPE:
-
High-density polyethylene
- BSH:
-
Bile salt hydrolase
- BAs:
-
Bile acids
- GIT:
-
Gastrointestinal tract
- SCFA:
-
Short-chain fatty acid
- LAB:
-
Lactic acid bacteria
- B. :
-
Bacillus
- WPI:
-
Whey protein isolate
- GA:
-
Gum arabic
- P. :
-
Pediococcus
- L. :
-
Lactobacillus, Lacticaseibacillus, Ligilactobacillus, Lactiplantibacillus, Limosilactobacillus
References
Liang B, Xing D (2023) The current and future perspectives of postbiotics. Probiotics Antimicrob Proteins 15:1626–1643
O’Callaghan A, van Sinderen D (2016) Bifidobacteria and their role as members of the human gut microbiota. Front Microbiol 7:925
Tirta GD, Martin L, Bani MD, Kho K, Pramanda IT, Pui LP et al (2023) Spray drying encapsulation of Pediococcus acidilactici at different inlet air temperatures and wall material ratios. Foods 12:165
Hills RD, Pontefract BA, Mishcon HR, Black CA, Sutton SC, Theberge CR (2019) Gut microbiome: profound implications for diet and disease. Nutrients 11(7):1613
De Simone C (2018) The unregulated probiotic market. Clin Gastroenterol Hepatol 17:809–817
Sanchez B, Delgad S, Blanco-Miguez A, Lourenco A, Gueimonde M, Margolles A (2017) Probiotics, gut microbiota, and their influence on host health and disease. Mol Nutr Food Res 61:1600240
Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D (2017) Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev 2:CD006095
Fiocco D, Longo A, Arena MP, Russo P, Spano G, Capozzi V (2020) How probiotics face food stress: they get by with a little help. Crit Rev Food Sci Nutr 60(9):1552–1580
Wang R, Sun R, Yang Y, Yang Y, He Y, Gong X et al (2023) Calcium ions enhanced freeze-drying and spray-drying resistance of Lactiplantibacillus plantarum LIP-1 by regulation of cell wall surface proteins and related enzymes expression. LWT 189:115460
Vorländer K, Pramann P, Kwade A, Finke JH, Kampen I (2023) Process and formulation parameters influencing the survival of Saccharomyces cerevisiae during spray drying and tableting. Int J Pharm 642:123100
Castro-López C, Romero-Luna HE, García HS, Vallejo-Cordoba B, González-Córdova AF, Hernández-Mendoza A (2023) Key stress response mechanisms of probiotics during their journey through the digestive system: a review. Probiotics Antimicrob Proteins 15(5):1250–1270
Rao NS, Ermann Lundberg L, Tomasson J, Tullberg C, Brink DP, Palmkron SB, van Niel EW, Håkansson S, Carlquist M (2023) Non-inhibitory levels of oxygen during cultivation increase freeze-drying stress tolerance in Limosilactobacillus reuteri DSM 17938. Front Microbiol 14:1152389
Kiepś J, Dembczyński R (2022) Current trends in the production of probiotic formulations. Foods 11(15):2330
Elisashvili V, Kachlishvili E, Chikindas ML (2019) Recent advances in the physiology of spore formation for bacillus probiotic production. Probiotics Antimicrob Proteins 11:731–747
Kaźmierczak Siedlecka K, Ruszkowski J, Fic M, Folwarski M, Wojciech Makarewicz W (2020) Saccharomyce boulardii CNCM I-745: a non bacterial microorganism used as probiotic agent in supporting treatment of selected diseases. Curr Microbiol 77:1987–1996
Sanhueza E, Paredes-Osses E, González CL, García A (2015) Effect of pH in the survival of Lactobacillus salivarius strain UCO_979C wild type and the pH acid acclimated variant. Electron J Biotechnol 18:343–346
Ortiz Camargo AR, Van Mastrigt O, Bongers RS, Ben-Amor K, Knol J, Smid EJ, Abee T (2023) Enhanced stress resistance of Bifidobacterium breve NRBB57 by induction of stress proteins at near zero growth rates. Benef Microbes 14:85–94
Bisson G, Maifreni M, Innocente N, Marino M (2023) Application of pre-adaptation strategies to improve the growth of probiotic Lactobacilli under food relevant stressful conditions. Food Funct 14:2128
Dailin DJ, Bing LY, Hanapi SZ, Mat SA, Yenn TW, Zailini SN, Çamkerten I, Sukmawati D, El Enshasy HA (2023) A review on exopolysaccharide production by Lactobacillus acidophilus and their techno-functional applications in food and pharmaceutical industry. Biosci Res 20(1):208–217
Dailin DJ, Elsayed EA, Malek RA, Hanapi SZ, Selvamani S, Ramli S, Sukmawati D, Sayyed RZ, El Enshasy HA (2020) Efficient kefiran production by Lactobacillus kefiranofacients ATCC 43761 in submerged cultivation: influence of osmotic stress and nonionic surfactants, and potential bioactivities. Arab J Chem 13:8513–8523
Ninomiya K, Matsuda K, Kawahata T, Kanaya T, Kohno M, Katakura Y, Asada M, Shioya S (2009) Effect of CO2 concentration on the growth and exopolysaccharide production of Bifidobacterium longum cultivated under anaerobic conditions. J Biosci Bioeng 107:535–537
Kiepś J, Olejnik A, Juzwa W, Dembczyński R (2023) Economic analysis of the production process of probiotics based on the biological and physiological parameters of the cells. Appl Sci 13(20):11541
Kim M, Nam D, Kim S, Im P, Choe J, Choi A (2018) Enhancement of viability, acid, and bile tolerance and accelerated stability in lyophilized Weissella cibaria JW15 with protective agents. Food Sci Nutr 6:1904–1913
Nguyen H-T, Turuong D-H, Kouhounde S, Lu S, Razafindralamo H, Delvigne F (2016) Biochemical engineering approaches for increasing viability and functionality of probiotic bacteria. Int J Mol Sci 17:867
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus. A review Proteomics 4:106–122
Ma J, Xu C, Liu F, Hou J, Shao H, Yu W (2021) Stress adaptation and cross-protection of Lactobacillus plantarum KLDS 1.0628. CYTA J Food 19:72–80
Min B, Kim K, Li V, Cho S, Kim H (2020) Changes in cell membrane and fatty acid composition of Streptococcus thermophilus in response to gradually increasing heat temperature. J Microbiol Biotechnol 30:739–748
Jonathan I, Devanthi PVP, Arham AGA, Crystalia AA, Ying CLS, Pramanda IT (2023) Effects of temperature shock on viability and stress-related gene expression in Pediococcus acidilactici, a probiotic lactic acid bacteria. In IOP Conference Series: Earth and Environmental Science 1255(1):012068 IOP Publishing
Wendel U (2022) Assessing viability and stress tolerance of probiotics—a review. Front Microbiol 12:818468
Succi M, Tremonte P, Pannella G, Tipaldi L, Cozzolino A, Romaniello R et al (2017) Pre-cultivation with selected prebiotics enhances the survival and the stress response of Lactobacillus rhamnosus strains in simulated gastrointestinal transit. Front Microbiol 8:1067
Bircher L, Geirnaert A, Hammes F, Lacroix C, Schwab C (2018) Effect of cryopreservation and lyophilization on viability and growth of strict anaerobic human gut microbes. Microb Biotechnol 11(4):721–733
Rishabh D, Athira A, Preetha R, Nagamaniammai G (2023) Freeze dried probiotic carrot juice powder for better storage stability of probiotic. J Food Sci Technol 60(3):916–924
Agriopoulou S, Tarapoulouzi M, Varzakas T, Jafari SM (2023) Application of encapsulation strategies for probiotics: from individual loading to co-encapsulation. Microorganisms 11(12):2896
Broeckx G, Vandenheuvel D, Claes IJJ, Lebeer S, Kiekens F (2016) Drying techniques of probiotic bacteria as an important step towards the development of novel pharmabiotics. Int J Pharmaceut 505:303–318
Obruca S, Sedlacek P, Krzyzanek V, Mravec F, Hrubanova K, Samek O et al (2016) Accumulation of poly(3-hydroxybutyrate) helps bacterial cells to survive freezing. PLoS ONE 11:e0157778
Aryaee H, Ariaii P, Zare D, Mirdamadi S, Naghizadeh Raeisi S (2023) Evaluation of the physicochemical characteristics of a blend fruit juice powder mixed with Lactiplantibacillus plantarum: a comparison of spray drying and freeze drying. J Food Process Preserv 2023:1–11
Alves NN, Messaoud GB, Desobry S, Costa JMC, Rodrigues S (2016) Effect of drying technique and feed flow rate on bacterial survival and physicochemical properties of a non-dairy fermented probiotic juice powder. J Food Eng 189:45–54
Khan FF, Sohail A, Ghazanfar S, Ahmad A, Riaz A, Abbasi KS et al (2023) Recent innovations in non-dairy prebiotics and probiotics: physiological potential, applications, and characterization. Probiotics Antimicrob Proteins 15(2):239–263
Kaur H, Ghosh M (2023) Probiotic fermentation enhances bioaccessibility of lycopene, polyphenols and antioxidant capacity of guava fruit (Psidium guajava L). J Agric Food Res 14:100704
Verón HE, Contreras L, Isla MI, Torres S (2023) Assessment of technological and functional features of Lactiplantibacillus and Fructobacillus strains isolated from Opuntia ficus-indica fruits. NFS Journal 31:110–122
Kaveh S, Hashemi SMB, Abedi E, Amiri MJ, Conte FL (2023) Bio-preservation of meat and fermented meat products by lactic acid bacteria strains and their antibacterial metabolites. Sustainability 15(13):10154
Dysin AP, Egorov AR, Godzishevskaya AA, Kirichuk AA, Tskhovrebov AG, Kritchenkov AS (2023) Biologically active supplements affecting producer microorganisms in food biotechnology: a review. Molecules 28(3):1413
Shoaei F (2022) Food health with increased probiotic survival during storage. prebiotics and probiotics—from food to health, 1st ed.; ItechOpen: London, UK, 151–172
Ejaz A, Afzaal M, Saeed F, Waliat S, Shah YA, Imran A et al (2023) Development and characterization of symbiotic microcapsules to enhance the viability of probiotic under stressed conditions. Int J Food Prop 26(2):2838–2853
Yeboah PJ, Ibrahim SA, Krastonov A (2023) A review of fermentation and the nutritional requirements for effective growth media for lactic acid bacteria. Food Sci Appl Biotechnol 6(2):215–240
Boontun C, Vatanyoopaisarn S, Hankla S, Kuraya E, Tamaki Y (2020) Modification of media using food-grade components for the fermentation of Bifidobacterium and Lactobacillus strains in large-scale bioreactors. Prep Biochem Biotechnol. https://doi.org/10.1080/10826068.2020.1861009. Online ahead of print.
Hellebois T, Tsevdou M, Soukoulis C (2020) Functionalizing and bio-preserving processed food products via probiotic and synbiotic edible films and coatings. Adv Food Nutr Res 94:161–221
Vénica CI, Wolf IV, Beret MV, Bergamini CV, Burns P, Binetti A et al (2023) Influence of commercial starter culture on fermentation dynamics and quality characteristics of yogurts obtained with different formulations. J Sci Food Agric 103(2):569–575
Gomand F, Borges F, Burgain J, Guerin J, Revol-Junelles AM, Gaiani C (2019) Food matrix design for effective lactic acid bacteria delivery. Annu Rev Food Sci Technol 10:285–310
Cheng Z, Yan X, Wu J, Weng P, Wu Z (2022) Effects of freeze drying in complex lyoprotectants on the survival, and membrane fatty acid composition of Lactobacillus plantarum L1 and Lactobacillus fermentum L2. Cryobiology 105:1–9
Meena P, Kishore N (2023) Synergistic effects of osmolytes on solvent exclusion and resulting protein stabilization: studies with sucrose, taurine and sorbitol individually and in combination. J Mol Liq 372:121175
Kumar CU, Suryavanshi U, SontakenV RPY, Sankhala RS, Swamy MJ et al (2022) Effect of sorbitol on alpha-crystallin structure and function. Biochemistry 87(2):131–140
Jena R, Choudhury PK (2023) Bifidobacteria in fermented dairy foods: a health beneficial outlook. Probiotics Antimicrob Proteins. https://doi.org/10.1007/s12602-023-10189-w. Epub ahead of print. PMID: 37979040.
Gao PP, Liu HQ, Ye ZW, Zheng QW, Zou Y, Wei T et al (2023) (2023) The beneficial potential of protein hydrolysates as prebiotic for probiotics and its biological activity: a review. Crit Rev Food Sci Nutr. https://doi.org/10.1080/10408398.2023.2260467. Epub ahead of print. PMID: 37811651.
Pereira EPR, da Graça JS, Ferreira BM, Balthazar CF, Xavier-Santos D, Bezerril FF et al (2023) What are the main obstacles to turning foods healthier through probiotics incorporation? A review of functionalization of foods by probiotics and bioactive metabolites. Food Res Int 176:113785. https://doi.org/10.1016/j.foodres.2023.113785. Epub 2023 Dec 2. PMID: 38163702.
Pena FL, Souza MC, Valle MCP, Bezerra RM, Rostagno MA, Antunes AE (2021) Probiotic fermented milk with high content of polyphenols: study of viability and bioaccessibility after simulated digestion. Int J Dairy Technol 74(1):170–180
Abesinghe AMNL, Priyashantha H, Prasanna PHP, Kurukulasuriya MS, Ranadheera CS, Vidanarachchi JK (2020) Inclusion of probiotics into fermented buffalo (Bubalus bubalis) milk: an overview of challenges and opportunities. Fermentation 6(4):121
Chang HM, Foo HL, Loh TC, Lim ETC, Abdul Mutalib NE (2021) Comparative studies of inhibitory and antioxidant activities, and organic acids compositions of postbiotics produced by probiotic Lactiplantibacillus plantarum strains isolated from Malaysian foods. Front Vet Sci 7:602280
Ansari F, Bahadori A, Samakkhah SA, Pirouzian HR, Pourjafar H (2023) Probiotic lactic acid bacteria: taxonomy, properties and benefits. Chapter 46. In SM Jafari et al. (eds.)Springer Nature Switzerland. Handbook Food Bioactive Ingredients pp. 1474–1483. https://books.google.gr/books?id=pAfXEAAAQBAJ&pg=PA1472&lpg=PA1472&dq=Pirouzian+HR,+Pourjafar+H+(2023)+Probiotic+Lactic+Acid+Bacteria+46.+Handbook+of+Food+Bioactive+Ingredients,+1473&source=bl&ots=JJdviymQdr&sig=ACfU3U01RY0BcUOJw_5cbUp4h7fDgI9kKg&hl=el&sa=X&ve
Dinkçi N, Akdeniz V, Akalin AS (2019) Survival of probiotics in functional foods during shelf life. In: Galanakis CM (ed) Food quality and shelf life. Elsevier The Netherlands, Amsterdam, pp 201–233
Vorländer K, Bahlmann L, Kwade A, Finke JH, Kampen I (2023) Effect of process parameters, protectants and carrier materials on the survival of yeast cells during fluidized bed granulation for tableting. Pharmaceutics 15(3):884
Wang H, Huang T, Liu K, Yu J, Yao G, Zhang W et al (2022) Protective effects of whey protein hydrolysate on Bifidobacterium animalis ssp. lactis Probio-M8 during freeze-drying and storage. J Dairy Sci 105(9):7308–7321
Jiménez-González O, Guerrero-Beltrán JÁ (2022) Microencapsulates by spray of Lacticaseibacillus rhamnosus GG from fermented whole or skimmed cow’s milk added with Mexican honeysuckle (Justicia spicigera) extract using mesquite gum as carrier agent. Heliyon 8(9):e10733-1-9
Echegaray N, Goksen G, Kumar M, Sharma R, Hassoun A, Lorenzo JM et al (2023) A critical review on protein-based smart packaging systems: understanding the development, characteristics, innovations, and potential applications. Crit Rev Food Sci Nutr 1–16
Misra S, Pandey P, Dalbhagat CG, Mishra HN (2022) Emerging technologies and coating materials for improved probiotication in food products: a review. Food Bioprocess Technol 15(5):998–1039
Cruz AGD, Castro WF, Faria JDAF, Bolini HMA, Celeghini RMDS, Raices RSL (2013) Stability of probiotic yogurt added with glucose oxidase in plastic materials with different permeability oxygen rates during the refrigerated storage. Food Res Int 51(2):723–728
Ballini A, Charitos IA, Cantore S, Topi S, Bottalico L, Santacroce L (2023) About functional foods: the probiotics and prebiotics state of art. Antibiotics 12(4):635
Grujović MŽ, Mladenović KG, Nikodijević DD, Čomić LR (2019) Autochthonous lactic acid bacteria—presentation of potential probiotics application. Biotechnol Lett 41:1319–1331
Ferrando V, Quiberoni A, Reinhemer J, Suárez V (2015) Resistance of functional Lactobacillus plantarum strains against food stress conditions. Food Microbiol 48:63–71
Haddaji N, Krifi B, Lagha R, Khouadja S, Bakhrouf A (2015) Effect of high temperature on viability of Lactobacillus casei and analysis of secreted and GroEL proteins profiles. J Bacteriol Res 7(3):29–35
Ferrando V, Quiberoni A, Reinheimer J, Suárez V (2016) Functional properties of Lactobacillus plantarum strains: a study in vitro of heat stress influence. Food Microbiol 54:154–161
Chen MJ, Tang HY, Chiang ML (2017) Effects of heat, cold, acid and bile salt adaptations on the stress tolerance and protein expression of kefir-isolated probiotic Lactobacillus kefiranofaciens M1. Food Microbiol 66:20–27
Hernández-Alcántara AM, Wacher C, Llamas MG, López P, Pérez-Chabela ML (2018) Probiotic properties and stress response of thermotolerant lactic acid bacteria isolated from cooked meat products. LWT 91:249–257
Kathiriya MR, Vekariya YV, Hati S (2023) Understanding the probiotic bacterial responses against various stresses in food matrix and gastrointestinal tract: a review. Probiotics Antimicrob Proteins 15(4):1032–1048. https://doi.org/10.1007/s12602-023-10104-3. Epub 2023 Jun 22. PMID: 37347421.
Molan K, Žgur Bertok D (2022) Small prokaryotic DNA-binding proteins protect genome integrity throughout the life cycle. Int J Mol Sci 23(7):4008
Chen W, Yu L, Shi Y (2019) Springer Nature Singapore Pte Ltd. and Science Press 2019. In: Chen W (ed) Lactic acid bacteria, omics and functional evaluation. pp 371–409. https://doi.org/10.1007/978-981-13-7832-4_11
Cichońska P, Kowalska E, Ziarno M (2023) The survival of psychobiotics in fermented food and the gastrointestinal tract: a review. Microorganisms 11(4):996
Louesdon S, Charlot-Rougé S, Tourdot-Maréchal R, Bouix M, Béal C (2015) Membrane fatty acid composition and fluidity are involved in the resistance to freezing of Lactobacillus buchneri R 1102 and Bifidobacterium longum R 0175. Microb Biotechnol 8(2):311–318
Pourjafar H, Ansari F, Sadeghi A, Samakkhah SA, Jafari SM (2023) Functional and health-promoting properties of probiotics’ exopolysaccharides; isolation, characterization, and applications in the food industry. Crit Rev Food Sci Nutr 63(26):8194–8225
Tripathi MK, Giri SK (2014) Probiotic functional foods: survival of probiotics during processing and storage. J Funct Foods 9:225–241
Barria C, Malecki M, Arraiano CM (2013) Bacterial adaptation to cold. Microbiology 159(Pt_12):2437–2443
Tsunetsugu-Yokota Y, Kobayahi-Ishihara M, Wada Y, Terahara K, Takeyama H, Kawana-Tachikawa A et al (2016) Homeostatically maintained resting naive CD4+ T cells resist latent HIV reactivation. Front Microbiol 7:1944
Mangiagalli M, Sarusi G, Kaleda A, Bar Dolev M, Nardone V, Vena VF et al (2018) Structure of a bacterial ice binding protein with two faces of interaction with ice. FEBS J 285(9):1653–1666
Polo L, Mañes-Lázaro R, Olmeda I, Cruz-Pio LE, Medina Á, Ferrer S (2017) Influence of freezing temperatures prior to freeze-drying on viability of yeasts and lactic acid bacteria isolated from wine. J Appl Microbiol 122(6):1603–1614
Guan NZ, Liu L (2020) Microbial response to acid stress: mechanisms and applications. Appl Microbiol Biotechnol 104:51–65
Perez HA, Bustos AY, Taranto MP, Frías MDLA, Ledesma AE (2018) Effects of lysozyme on the activity of ionic of fluoroquinolone species. Molecules 23(4):741
Vila T, Rizk AM, Sultan AS, Jabra-Rizk MA (2019) The power of saliva: antimicrobial and beyond. PLoS Pathog 15(11):e1008058
Han S, Lu Y, Xie J, Fei Y, Zheng G, Wang Z et al (2021) Probiotic gastrointestinal transit and colonization after oral administration: a long journey. Front Cell Infect 11:609722
Sun Y (2016) F1F0-ATPase functions under markedly acidic conditions in bacteria. In: Chakraborti S, Dhalla NS (eds) Regulation of Ca2+-ATPases, V-ATPases and F-ATPases. Springer International Publishing, Cham, Switzerland, pp 459–468
Papadimitriou K, Alegría Á, Bron PA, de Angelis M, Gobbetti M, Kleerebezem M et al (2016) Stress physiology of lactic acid bacteria. Microbiol Mol Biol Rev 80(3):837–890
Ahire JJ, Jakkamsetty C, Kashikar MS, Lakshmi SG, Madempudi RS (2021) In vitro evaluation of probiotic properties of Lactobacillus plantarum UBLP40 isolated from traditional indigenous fermented food. Probiotics Antimicrob Proteins 13(5):1413–1424
Sesín AA, Paz JJC, Ledesma AE, Taranto MP, Bustos AY (2023) Probiotic characterization of lactic acid bacteria from artisanal goat cheese for functional dairy product development. Braz J Food Technol 26:e2023024
Chen T, Wang L, Li Q, Long Y, Lin Y, Yin J et al (2020) Functional probiotics of lactic acid bacteria from Hu sheep milk. BMC Microbiol 20(1):1–12
Bustos AY, de Valdez GF, Fadda S, Taranto MP (2018) New insights into bacterial bile resistance mechanisms: the role of bile salt hydrolase and its impact on human health. Food Res Int 112:250–262
Mbye M, Baig MA, AbuQamar SF, El-Tarabily KA, Obaid RS, Osail TM et al (2020) Updates on understanding of probiotic lactic acid bacteria responses to environmental stresses and highlights on proteomic analyses. Compr Rev Food Sci Food Saf 19(3):1110–1124
Sensoy I (2021) A review on the food digestion in the digestive tract and the used in vitro models. Curr Res Food Sci 4:308–319
Ouwehand AC, Tölkkö S, Salminen S (2001) The effect of digestive enzymes on the adhesion of probiotic bacteria in vitro. J Food Sci 66(6):856–859
Chai LN, Wu H, Wang XJ, He LJ, Guo CF (2023) The mechanism of antimicrobial activity of conjugated bile acids against lactic acid bacilli. Microorganisms 11(7):1823
Prete R, Long SL, Gallardo AL, Gahan CG, Corsetti A, Joyce SA (2020) Beneficial bile acid metabolism from Lactobacillus plantarum of food origin. Sci Rep 10(1):1165
Collins SL, Stine JG, Bisanz JE, Okafor CD, Patterson AD (2023) Bile acids and the gut microbiota: metabolic interactions and impacts on disease. Nat Rev Microbiol 21(4):236–247
Di Gregorio MC, Cautela J, Galantini L (2021) Physiology and physical chemistry of bile acids. Int J Mol Sci 22:1780
Taranto MP, Perez-Martinez G, de Valdez GF (2006) Effect of bile acid on the cell membrane functionality of lactic acid bacteria for oral administration. Res Microbiol 157(8):720–725
Bi J, Liu S, Du G, Chen J (2016) Bile salt tolerance of Lactococcus lactis is enhanced by expression of bile salt hydrolase thereby producing less bile acid in the cells. Biotechnol Lett 38:659–665
Benarroch JM, Asally M (2020) The microbiologist’s guide to membrane potential dynamics. Trends Microbiol 28:304–314
Kurdi P, Kawanishi K, Mizutani K, Yokota A (2006) Mechanism of growth inhibition by free bile acids in lactobacilli and bifidobacteria. J Bacteriol 188:1979–1986
Bustos AY, Raya R, de Valdez GF, Taranto MP (2011) Efflux of bile acids in Lactobacillus reuteri is mediated by ATP. Biotechnol Lett 33:2265–2269
Bustos AY, Saavedra L, de Valdez GF, Raya RR, Taranto MP (2012) Relationship between bile salt hydrolase activity, changes in the internal pH and tolerance to bile acids in lactic acid bacteria. Biotechnol Lett 34:1511–1518
Cremers CM, Knoefler D, Vitvitsky V, Banerjee R, Jakob U (2014) Bile salts act as effective protein-unfolding agents and instigators of disulfide stress in vivo. Proc Natl Acad Sci 111(16):E1610–E1619
Bustos AY, De los Angeles Frias M, Ledesma AE (2022) Biophysical and structural insights in α-amylase and bile acids interaction. ChemistrySelect 7(14):e202103198
Wang RM, Li N, Zheng K, Hao JF (2018) Enhancing acid tolerance of the probiotic bacterium Lactobacillus acidophilus NCFM with trehalose. FEMS Microbiol Lett 365(19):fny217
Wu C, Zhang J, Wang M, Du G, Chen J (2012) Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J Ind Microbiol Biotechnol 39(7):1031–1039
Gómez Zavaglia A, Kociubinski G, Pérez P, Disalvo E, De Antoni G (2002) Effect of bile on the lipid composition and surface properties of bifidobacteria. J Appl Microbiol 93(5):794–799
Kato S, Tobe H, Matsubara H, Sawada M, Sasaki Y, Fukiya S et al (1864) (2019) The membrane phospholipid cardiolipin plays a pivotal role in bile acid adaptation by Lactobacillus gasseri JCM1131T. Biochim Biophys Acta Mol Cell Biol Lipids 3:403–412
Shimizu K, Ito M, Katto M, Takada T, Oana K, Makino H et al (2023) Identification of genes essential for bile acid resistance in the probiotic Lacticaseibacillus paracasei strain Shirota. Lett Appl Microbiol 76(6):ovad062
Bustos AY, de Valdez GF, Raya R, de Almeida AM, Fadda S, Taranto MP (2015) Proteomic analysis of the probiotic Lactobacillus reuteri CRL1098 reveals novel tolerance biomarkers to bile acid-induced stress. Food Res Int 77:599–607
Ali SA, Singh P, Tomar SK, Mohanty AK, Behare P (2020) Proteomics fingerprints of systemic mechanisms of adaptation to bile in Lactobacillus fermentum. J Proteomics 213:103600
Wang GQ, Pu J, Yu XQ, Xia YJ, Ai LZ (2020) Influence of freezing temperature before freeze-drying on the viability of various Lactobacillus plantarum strains. J Dairy Sci 103(4):3066–3075
Bagon BB, Oh JK, Valeriano VDV, Pajarillo EAB, Kang DK (2021) Exploring the bile stress response of Lactobacillus mucosae LM1 through exoproteome analysis. Molecules 26(18):5695
Bagon B, Valeriano VDV, Oh JK, Pajarillo EAB, Lee JY, Kang DK (2021) Exoproteome perspective on the bile stress response of Lactobacillus johnsonii. Proteomes 9:10
Wei Y, Gao J, Liu D, Li Y, Liu W (2019) Adaptational changes in physiological and transcriptional responses of Bifidobacterium longum involved in acid stress resistance after successive batch cultures. Microb Cell Factories 18:1–10
Sánchez B, Champomier-Verges MC, Collado MDC, Anglade P, Baraige F, Sanz Y et al (2007) Low-pH adaptation and the acid tolerance response of Bifidobacterium longum biotype longum. Appl Environ Microbiol 73(20):6450–6459
Jin J, Qin Q, Guo H, Liu S, Ge S, Zhang H et al (2015) Effect of pre-stressing on the acid-stress response in Bifidobacterium revealed using proteomic and physiological approaches. PLoS ONE 10(2):e0117702
Baig MA, Turner MS, Liu SQ, Al-Nabulsi AA, Shah NP, Ayyash MM (2021) Potential probiotic Pediococcus pentosaceus M41 modulates its proteome differentially for tolerances against heat, cold, acid, and bile stresses. Front Microbiol 12:731410
Jung S, Lee JH (2020) Characterization of transcriptional response of Lactobacillus plantarum under acidic conditions provides insight into bacterial adaptation in fermentative environments. Sci Rep 10(1):19203
Koponen J, Laakso K, Koskenniemi K, Kankainen M, Savijoki K, Nyman TA, Varmanen P (2012) Effect of acid stress on protein expression and phosphorylation in Lactobacillus rhamnosus GG. J Proteom 75:1357–1374
Broadbent JR, Larsen RL, Deibel V, Steele JL (2010) Physiological and transcriptional response of Lactobacillus casei ATCC 334 to acid stress. J Bacteriol 192:2445–2458
Ruiz L, Zomer A, O’Connell-Motherway M, van Sinderen D, Margolles A (2012) Discovering novel bile protection systems in Bifidobacterium breve UCC2003 through functional genomics. App Environ Microbiol 78:1123–1131
Hagi T, Geerlings SY, Nijsse B, Belzer C (2020) The effect of bile acids on the growth and global gene expression profiles in Akkermansia muciniphila. Appl Microbiol Biotechnol 104:10641–10653
Whitehead K, Versalovic J, Roos S, Britton RA (2008) Genomic and genetic characterization of the bile stress response of probiotic Lactobacillus reuteri ATCC 55730. Appl Environ Microbiol 74(6):1812–1819
Ruiz L, Margolles A, Sánchez B (2013) Bile resistance mechanisms in Lactobacillus and Bifidobacterium. Front Microbiol 4:396
Noriega L, Cuevas I, Margolles A, Clara G (2006) Deconjugation and bile salts hydrolase activity by Bifidobacterium strains with acquired resistance to bile. Int Dairy J 16(8):850–855
Yuan J, Wang B, Sun Z, Bo X, Yuan X, He X et al (2007) Analysis of hostinducing proteome changes in Bifidobacterium longum NCC2705 grown in vivo. J Proteome Res 7(01):375–385
He L, He T, Farrar S, Ji L, Liu T, Ma X (2017) Antioxidants maintain cellular redox homeostasis by elimination of reactive oxygen species. Cell Physiol Biochem 44(2):532–553
Sohlenkamp C (2017) Membrane homeostasis in bacteria upon PH challenge. In Biogenesis of fatty acids, lipids and membranes. Springer International Publishing: Cham, Switzerland, pp 1–13
Siegumfeldt H, Bjorn Rechinger K, Jakobsen M (2000) Dynamic changes of intracellular pH in individual lactic acid bacterium cells in response to a rapid drop in extracellular pH. Appl Environ Microbiol 66(6):2330–2335
Chand D, Avinash VS, Yadav Y, Pundle AV, Suresh CG (1861) Ramasamy S (2017) Molecular features of bile salt hydrolases and relevance in human health. Biochim Biophys Acta 1(1 Pt A):2981–2991
Taranto MP, Fernandez Murga ML, Lorca G, Valdez GF (2003) Bile salts and cholesterol induce changes in the lipid cell membrane of Lactobacillus reuteri. J Appl Microbiol 95:86–91
Hu B, Tian F, Wang G, Zhang Q, Zhao J, Zhang H et al (2015) Enhancement of bile resistance in Lactobacillus plantarum strains by soy lecithin. Lett Appl Microbiol 61(1):13–19
Gaucher F, Bonnassie S, Rabah H, Marchand P, Blanc P, Jeantet R et al (2019) Review: Adaptation of beneficial Propionibacteria, Lactobacilli, and Bifidobacteria improves tolerance toward technological and digestive stresses. Front Microbiol 10:841
Begley M, Gahan CG, Hill C (2005) The interaction between bacteria and bile. FEMS Microbiol Rev 29(4):625–651
Jones BV, Begley M, Hill C, Gahan CG, Marchesi JR (2008) Functional and comparative metagenomic analysis of bile salt hydrolase activity in the human gut microbiome. Proc Natl Acad Sci 105(36):13580–13585
O’Flaherty S, Briner Crawley A, Theriot CM, Barrangou R (2018) The Lactobacillus bile salt hydrolase repertoire reveals niche-specific adaptation. Msphere 3(3):10–1128
Hamon E, Horvatovich P, Izquierdo E, Bringel F, Marchioni E, Aoudé-Werner D et al (2011) Comparative proteomic analysis of Lactobacillus plantarum for the identification of key proteins in bile tolerance. BMC Microbiol 11(1):63
Bøhle LA, Færgestad EM, Veiseth-Kent E, Steinmoen H, Nes IF, Eijsink VG et al (2010) Identification of proteins related to the stress response in Enterococcus faecalis V583 caused by bovine bile. Proteome Sci 8(1):1–12
Lee K, Lee HG, Choi YJ (2008) Proteomic analysis of the effect of bile salts on the intestinal and probiotic bacterium Lactobacillus reuteri. J Biotech 137(1–4):14–19
Fonseca F, Béal C, Corrieu G (2001) Operating conditions that affect the resistance of lactic acid bacteria to freezing and frozen storage. Cryobiology 43(3):189–198
Velly H, Fonseca F, Passot S, Delacroix-Buchet A, Bouix M (2014) Cell growth and resistance of Lactococcus lactis subsp. lactis TOMSC 161 following freezing, drying and freeze-dried storage are differentially affected by fermentation conditions. J Appl Microbiol 117(3):729–740
Huang S, Rabah H, Jardin J, Briard-Bion V, Parayre S, Maillard M-B et al (2016) Hyperconcentrated sweet whey: a new culture medium that enhances Propionibacterium freudenreichii stress tolerance. Appl Environ Microbiol 82(15):4641–4651
Correa Deza MA, Salva S, Grillo-Puertas M, Font GM, Gerez CL (2023) Effect of culture parameters on the heat tolerance and inorganic polyphosphate accumulation by Lacticaseibacillus rhamnosus CRL1505, a multifunctional bacterium. World J Microbiol Biotechnol 39(7):182
Ai Z, Lv X, Huang S, Liu G, Sun X, Chen H et al (2017) The effect of controlled and uncontrolled pH cultures on the growth of Lactobacillus delbrueckii subsp. Bulgaricus LWT 77:269–275
Zotta T, Ricciardi A, Ciocia F, Rossano R, Parente E (2008) Diversity of stress responses in dairy thermophilic streptococci. Int J Food Microbiol 124:34–42
Peighambardoust S, Tafti AG, Hesari J (2011) Application of spray drying for preservation of lactic acid starter cultures: a review. Trends Food Sci Technol 22(5):215–224
Alcántara C, Coll-Marqués JM, Jadán-Piedra C, Vélez D, Devesa V, Zúñiga M et al (2018) Polyphosphate in Lactobacillus and its link to stress tolerance and probiotic properties. Front Microbiol 9:1944
Morgan CA, Herman N, White P, Vesey G (2006) Preservation of micro-organisms by drying; a review. J Microbiol Methods 66(2):183–193
Correa Deza MA, Díaz Vergara L, Salva S, Montenegro MA, Font de Valdez G, Gerez CL (2021) Inorganic additive improves the survival of the probiotic Lacticaseibacillus rhamnosus CRL1505 during spray drying, rehydration, and storage. Curr Microbiol 78:3863–3871
Lindner JDD, Canchaya C, Zhang Z, Neviani E, Fitzgerald GF, van Sinderen D et al (2007) Exploiting bifidobacterium genomes: the molecular basis of stress response. Int J Food Microbiol 120(1–2):13–24
Sleator RD, Hill C (2002) Bacterial osmoadaptation: the role of osmolytes in bacterial stress and virulence. FEMS Microbiol Rev 26(1):49–71
Corcoran BM, Ross RP, Fitzgerald GF, Dockery P, Stanton C (2006) Enhanced survival of GroESL-overproducing Lactobacillus paracasei NFBC 338 under stressful conditions induced by drying. Appl Environ Microbiol 72(7):5104–5107
Sleator RD, Gahan CGM, Hill C (2003) A postgenomic appraisal of osmotolerance in Listeria monocytogenes. Appl Environ Microbiol 69(1):1–9
Sleator RD, Gahan CGM, Abee T, Hill C (1999) Identification and disruption of BetL, a secondary glycine betaine transport system linked to the salt tolerance of Listeria monocytogenes LO28. Appl Environ Microbiol 65(5):2078–2083
Sheehan VM, Sleator RD, Fitzgerald GF, Hill C (2006) Heterologous expression of BetL, a betaine uptake system, enhances the stress tolerance of Lactobacillus salivarius UCC118. Appl Environ Microbiol 72(3):2170–2177
Zotta T, Ricciardi A, Ianniello RG, Parente E, Reale A, Rossi F et al (2014) Assessment of aerobic and respiratory growth in the Lactobacillus casei group. PLoS ONE 9:e99189
Imlay JA (2019) Where in the world do bacteria experience oxidative stress? Env Microbiol 21:521–530
Zotta T, Parente E, Ricciardi A (2017) Aerobic metabolism in the genus Lactobacillus: impact on stress response and potential applications in the food industry. J Appl Microbiol 122:857–869
Zotta T, Ricciardi A, Ianniello RG, Storti LV, Glibota NA, Parente E (2018) Aerobic and respirative growth of heterofermentative lactic acid bacteria: a screening study. Food Microbiol 76:117–127
Pedersen MB, Gaudu P, Lechardeur D, Petit MA, Gruss A (2012) Aerobic respiration metabolism in lactic acid bacteria and uses in biotechnology. Annu Rev Food Sci Technol 3:37–58
Zhu Z, Yang P, Wu Z, Zhang J, Du G (2019) Systemic understanding of Lactococcus lactis response to acid stress using transcriptomics approaches. J Ind Microbiol Biotechnol 46:1621–1629
In Seong C, Seung Hee K, Ho Myeong K, Ho Hyun C, Kwang Ho L, Jung Eun Y et al (2019) Shelf-life extension of freeze-dried Lactobacillus brevis WiKim0069 using supercooling pretreatment. LWT - Food Sci Technol 112:108230
Derzelle S, Hallet B, Ferain T, Delcour J, Hols P (2003) Improved adaptation to cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl Environ Microbiol 69:4285–4290
Song S, Bae DW, Lim K, Oh GMWS (2014) Cold stress improves the ability of Lactobacillus plantarum L67 to survive freezing. Int J Food Microbiol 191:135–143
Carvalho AS, Silva J, Ho P, Teixeira P, Malcata FX, Gibbs P (2004) Relevant factors for the preparation of freeze-dried lactic acid bacteria. Int Dairy J 14:835–847
Stefanello RF, Nabeshima EH, Iamanaka BT, Ludwig A, Martins Fries LL, Bernardi AO et al (2019) Survival and stability of Lactobacillus fermentum and Wickerhamomyces anomalus strains upon lyophilisation with different cryoprotectant agents. Food Res Int 115:90–94
Sun HY, Zhang MH, Liu YK, Wang Y, Chen YY, Guan WY et al (2021) Improved viability of Lactobacillus plantarum embedded in whey protein concentrate/pullulan/trehalose hydrogel during freeze drying. Carbohydr Polym 260:10
Kanimozhi NV, Sukumar M (2023) Effect of different cryoprotectants on the stability and survivability of freeze dried probiotics. Food Chem Adv 3:100428
Di L, Ma W, Kang W, Huang Y, Wu Z, Yin B et al (2023) Synergistic combination of cryoprotectants for high freeze-dried survival rate and viable cell counts of Streptococcus thermophilus. Dry Technol 41(9):1444–1453
Van de Guchte M, Serror P, Chervaux C, Smokvina T, Ehrlich SD, Maguin E (2002) Stress responses in lactic acid bacteria. In Lactic acid bacteria: genetics, metabolism and applications: Proceedings of the seventh Symposium on lactic acid bacteria: genetics, metabolism and applications, 1–5 September 2002, Springer, Egmond aan Zee, the Netherlands (pp 187–216).
Santivarangkna C, Kulozik U, Foerst P (2008) Inactivation mechanisms of lactic acid starter cultures preserved by drying processes. J Appl Microbiol 105(1):1–13
Perdana J, Bereschenko L, Fox MB, Kuperus JH, Kleerebezem M, Boom RM et al (2013) Dehydration and thermal inactivation of Lactobacillus plantarum WCFS1: comparing single droplet drying to spray and freeze drying. Food Res Int 54(2):1351–1359
Santivarangkna C, Kulozik U, Foerst P (2007) Alternative drying processes for the industrial preservation of lactic acid starter cultures. Biotechnol Prog 23(2):302–315
Leylak C, Özdemir KS, Gurakan GC, Ogel ZB (2021) Optimisation of spray drying parameters for Lactobacillus acidophilus encapsulation in whey and gum arabic: its application in yoghurt. Int Dairy J 112:104865
Sharifi S, Rezazad-Bari M, Alizadeh M, Almasi H, Amiri S (2021) Use of whey protein isolate and gum arabic for the co-encapsulation of probiotic Lactobacillus plantarum and phytosterols by complex coacervation: enhanced viability of probiotic in Iranian white cheese. Food Hydrocoll 113:106496
Fu N, Chen XD (2011) Towards a maximal cell survival in convective thermal drying processes. Food Res Int 44:1127–1149
Gardiner GE, O’Sullivan E, Kelly J, Auty MAE, Fitzgerald GF, Collins JK et al (2000) Comparative survival rates of human-derived probiotic Lactobacillus paracasei and L salivarius strains during heat treatment and spray drying. Appl Environ Microbiol 66:2605–2615
Ananta E, Volkert M, Knorr D (2005) Cellular injuries and storage stability of spray dried Lactobacillus rhamnosus GG. Int Dairy J 15:399–409
Sunny-Roberts EO, Knorr D (2009) The protective effect of monosodium glutamate on survival of Lactobacillus rhamnosus GG and Lactobacillus rhamnosus E-97800 (E800) strains during spray-drying and storage in trehalose-containing powders. Int Dairy J 19:209–214
Riveros B, Ferrer J, Borquez R (2009) Spray drying of a vaginal probiotic strain of Lactobacillus acidophilus. Dry Technol 27(1):123–132
Zelaya H, Alvarez S, Kitazawa H, Villena J (2016) Respiratory antiviral immunity and immunobiotics: beneficial effects on inflammation-coagulation interaction during influenza virus infection. Front Immunol 7:633
Oldenhof H, Wolkers WF, Fonseca F, Passot S, Marin M (2005) Effect of sucrose and maltodextrin on the physical properties and survival of air-dried Lactobacillus bulgaricus: an in situ Fourier transform infrared spectroscopy study. Biotechnol Prog 21(3):885–892
Correa Deza MA, Grillo-Puertas M, Salva S, Rapisarda VA, Gerez CL, Font de Valdez G (2017) Inorganic salts and intracellular polyphosphate inclusions play a role in the thermotolerance of the immunobiotic Lactobacillus rhamnosus CRL 1505. PLoS ONE 12(6):e0179242
Gao X, Kong J, Zhu H, Mao B, Cui S, Zhao J (2022) Lactobacillus, Bifidobacterium and Lactococcus response to environmental stress: mechanisms and application of cross-protection to improve resistance against freeze-drying. J Appl Microbiol 132(2):802–821
Conde-Islas AÁ, Jiménez-Fernández M, Cantú-Lozano D, Urrea-García GR, Luna-Solano G (2019) Effect of the freeze-drying process on the physicochemical and microbiological properties of Mexican kefir grains. Processes 7(3):127
Lapsiri W, Bhandari B, Wanchaitanawong P (2012) Viability of Lactobacillus plantarum TISTR 2075 in different protectants during spray drying and storage. Dry Technol 30:1407–1412
Schutyser AIM, Perdana J, Boom RM (2012) Single droplet drying for optimal spray drying of enzymes and probiotics. Trends Food Sci Technol 27(2):73–82
Ahi M, Hatamipour MS, Goodarzi A (2010) Optimization of leaving activity of baker’s yeast during the spray-drying process. Dry Technol 28(4):490–494
Ying DY, Sun J, Sanguansri L, Weerakkody R, Augustin MA (2012) Enhanced survival of spray-dried microencapsulated Lactobacillus rhamnosus GG in the presence of glucose. J Food Eng 109:597–602
Kothari D, Patel S, Kim SK (2019) Probiotic supplements might not be universally-effective and safe: a review. Biomed Pharmacother 111:537–547
Nataraj BH, Ali SA, Behare PV, Yadav H (2020) Postbioticsparabiotics: the new horizons in microbial biotherapy and functional foods. Microb Cell Fact 19:168
Barros CP, Guimaraes JT, Esmerino EA, Duarte MCK, Silva MC, Silva R, Ferreira BM, Sant’Ana AS, Freitas MQ, Cruz AG (2020) Paraprobiotics and postbiotics: concepts and potential applications in dairy products. Curr Opin Food Sci 32:1–8
Park SW, Choi YH, Gho JY, Kang GA, Kang SS (2024) Synergistic inhibitory effect of Lactobacillus cell lysates and butyrate on poly I:C-induced IL-8 production in human intestinal epithelial cells. Probiotics Antimicrob Proteins 16:1–12. https://doi.org/10.1007/s12602-023-10042-0
Hosseini H, Abbasi A, Sabahi S, Akrami S, Yousefi-Avarvand A (2023) Assessing the potential biological activities of postbiotics derived from Saccharomyces cerevisiae: an in vitro study. Probiotics and Antimicrobial Proteins. https://doi.org/10.1007/s12602-023-10117-y
Sabahi S et al (2023) Postbiotics as the new frontier in food and pharmaceutical research. Crit Rev Food Sci Nut 63(26):8875–8402. https://doi.org/10.1080/10408398.2022.2056727. Epub 2022 Mar 29. PMID: 35348016.
Abbasi A et al (2024) A critical review on the gluten-induced enteropathy/celiac disease: gluten-targeted dietary and nondietary therapeutic approaches. Food Rev Intern 40(3):883–923. https://doi.org/10.1080/87559129.2023.2202405
Ozma MA, Abbasi A, Sabahi S (2022) Characterization of postbiotics derived from Lactobacillus paracasei ATCC 55544 and its application in Malva sylvestris seed mucilage edible coating to the improvement of the microbiological, and sensory properties of lamb meat during storage. Biointerface Res Appl Chem 13(3):267–1-13
Abbasi A, Saadat TR, Saadat YR (2022) Microbial exopolysaccharides–β-glucans–as promising postbiotic candidates in vaccine adjuvants. Intern J Biol Macromol 223(Pt A):346–361. https://doi.org/10.1016/j.ijbiomac.2022.11.003. Epub 2022 Nov 5. PMID: 36347372
Abbasi A, Sheykhsaran E, Kafil HS (2021) Postbiotics: science, technology and applications. Bentham Sci Pub
Moradi M et al (2020) Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr Rev Food Sci Food Saf 19(6):3390–3415
Funding
Open access funding provided by HEAL-Link Greece. The authors did not receive support from any organization for the submitted work.
Author information
Authors and Affiliations
Contributions
All authors reviewed, read, and agreed to the published version of the manuscript and contributed equally.
Corresponding author
Ethics declarations
Ethics Approval
This article does not contain any studies with human or animal subjects.
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Bustos, A.Y., Taranto, M.P., Gerez, C.L. et al. Recent Advances in the Understanding of Stress Resistance Mechanisms in Probiotics: Relevance for the Design of Functional Food Systems. Probiotics & Antimicro. Prot. (2024). https://doi.org/10.1007/s12602-024-10273-9
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-024-10273-9