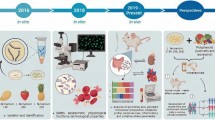
Abstract
A new biotherapeutic strategy involves the use of microbial bioactive substances (postbiotics) that exhibit optimum compatibility and intimate contact with the immune system of the host. This study was aimed at investigating the potential biological activities of postbiotics derived from Saccharomyces cerevisiae (PTCC 5269) (PSC) under in vitro circumstances. Based on the outcomes, the synthesized PSC possessing a high level of phenolic (102.46 ± 0.25 mg GAE/g) and flavonoid (19.87 ± 75.32 mg QE/g) content demonstrated significant radical scavenging activity (87.34 ± 0.56%); antibacterial action towards Listeria monocytogenes, Streptococcus mutans, Salmonella typhi, and Escherichia coli (in order of effectiveness) in both in vitro and food models (whole milk and ground meat); probiotics' growth-promoting activity in the fermentation medium; α-glucosidase enzyme-inhibiting and cholesterol-lowering properties in a concentration- and pH-dependent manner; reduction in the cell viability (with the significant IC50 values of 34.27 and 23.58 μg/mL after 24 and 48 h, respectively); suppressed the initial (G0/G1) phase of the cell's division; induced apoptosis; and increased the expression of PTEN gene, while the IkB, RelA, and Bcl-XL genes indicated diminished expression in treated SW480 cancer cells. These multiple health-promoting functions of PSC can be extended to medical, biomedical, and food scopes, as novel biotherapeutic approaches, in order to design efficient and optimized functional food formulations or/and supplementary medications to use as adjuvant agents for preventing or/and treating chronic/acute disorders.







Similar content being viewed by others
Data Availability
The data presented in this study are available on request from the corresponding author.
Abbreviations
- PSC:
-
Postbiotics derived from Saccharomyces cerevisiae
- QS:
-
Quorum sensing
- YPD:
-
Yeast peptone dextrose
- YMB:
-
Yeast malt broth
- ABTS:
-
2,2′-Azino-bis [3-ethylbenzothiazoline-6-sulfonic acid] diammonium salt
- MHA:
-
Mueller Hinton agar
- MHB:
-
Mueller Hinton broth
- SDA:
-
Sabouraud dextrose agar
- MSA:
-
Mannitol salt agar
- EMB:
-
Eosin methylene blue
- PCA:
-
Plate count agar
- BHI:
-
Brain heart infusion
- LB:
-
Luria Bertani
- CT-SMAC:
-
Cefixime tellurite sorbitol MacConkey
- CFS:
-
Cell-free supernatant
- GAE:
-
Gallic acid equivalent
- QE:
-
Quercetin equivalent
- DDA:
-
Disc diffusion agar
- MIC:
-
Minimum inhibitory concentration
- MBC:
-
Minimum bactericidal concentration
- WDA:
-
Well diffusion agar
- MEC:
-
Minimal effective concentration
- pNPG:
-
4-Nitrophenly α-D-glucopyranoside
- 5-FU:
-
5–Fluorouracil
- PBS:
-
Phosphate-buffered saline
- PI:
-
Propidium iodide
- ANOVA:
-
One-way analysis of variance
- ROS:
-
Reactive oxygen species
- AFB1 :
-
Aflatoxin B1
- CDC:
-
Centers for Disease Control and Prevention
References
Rajakovich LJ, Balskus EP (2019) Metabolic functions of the human gut microbiota: the role of metalloenzymes. Nat Prod Rep 36(4):593–625
Bhardwaj S et al (2021) Growing emergence of drug-resistant Pseudomonas aeruginosa and attenuation of its virulence using quorum sensing inhibitors: a critical review. Iran J Basic Med Sci 24(6):699
Sabahi S et al (2022) Postbiotics as the new frontier in food and pharmaceutical research. Crit Rev Food Sci Nut 1–28
Abbasi A et al (2023) A critical review on the gluten-induced enteropathy/celiac disease: gluten-targeted dietary and non-dietary therapeutic approaches. Food Rev Intern 1–41
Ozma MA, Abbasi A, Sabahi S (2022) Characterization of postbiotics derived from Lactobacillus paracasei ATCC 55544 and its application in Malva sylvestris seed mucilage edible coating to the improvement of the microbiological, and sensory properties of lamb meat during storage. Biointerface Res Appl Chem 13(3)
Abbasi A, Saadat TR, Saadat YR (2022) Microbial exopolysaccharides–β-glucans–as promising postbiotic candidates in vaccine adjuvants. Intern J Biol Macromole
Abbasi A, Sheykhsaran E, Kafil HS (2021) Postbiotics: science, technology and applications. Bentham Sci Pub
Moradi M et al (2020) Postbiotics produced by lactic acid bacteria: the next frontier in food safety. Compr Rev Food Sci Food Saf 19(6):3390–3415
Evers MS et al (2023) Thiamine and biotin: relevance in the production of volatile and non-volatile compounds during Saccharomyces cerevisiae alcoholic fermentation in synthetic grape must. Foods 12. https://doi.org/10.3390/foods12050972
Rahbar Saadat Y et al (2020) Modulatory role of exopolysaccharides of Kluyveromyces marxianus and Pichia kudriavzevii as probiotic yeasts from dairy products in human colon cancer cells. J Funct Foods 64:103675
Abeysekera W, Premakumara G, Ratnasooriya W (2013) In vitro antioxidant properties of leaf and bark extracts of ceylon cinnamon (Cinnamomum zeylanicum Blume)
Nantitanon W, Chowwanapoonpohn S, Okonogi S (2007) Antioxidant and antimicrobial activities of Hyptis suaveolens essential oil. Sci Pharm 75(1):35–54
Sabahi S, Abbasi A, Mortazavi SA (2022) Characterization of cinnamon essential oil and its application in Malva sylvestris seed mucilage edible coating to the enhancement of the microbiological, physicochemical and sensory properties of lamb meat during storage. J Appl Microbiol 133(2):488–502
Hartmann HA, Wilke T, Erdmann R (2011) Efficacy of bacteriocin-containing cell-free culture supernatants from lactic acid bacteria to control Listeria monocytogenes in food. Int J Food Microbiol 146(2):192–199
Wang K et al (2014) Characterization of a novel exopolysaccharide with antitumor activity from Lactobacillus plantarum 70810. Int J Biol Macromol 63:133–139
Kazeem M, Adamson J, Ogunwande I (2013) Modes of inhibition of α-amylase and α-glucosidase by aqueous extract of Morinda lucida Benth leaf. BioMed Res Intern
Soh H-S, Kim C-S, Lee S-P (2003) A new in vitro assay of cholesterol adsorption by food and microbial polysaccharides. J Med Food 6(3):225–230
Faghfoori MH et al (2020) Anticancer effect of X-Ray triggered methotrexate conjugated albumin coated bismuth sulfide nanoparticles on SW480 colon cancer cell line. Int J Pharm 582:119320
Pang H et al (2021) Exosomes derived from colon cancer cells and plasma of colon cancer patients promote migration of SW480 cells through Akt/mTOR pathway. Pathol Res Prac 222
Abbasi A et al (2023) Antigenotoxicity and cytotoxic potentials of cell-free supernatants derived from Saccharomyces cerevisiae var. boulardii on HT-29 human colon cancer cell lines. Probiot Antimicro Prot 1–13
Vahed SZ et al (2017) Leuconostoc mesenteroides-derived anticancer pharmaceuticals hinder inflammation and cell survival in colon cancer cells by modulating NF-κB/AKT/PTEN/MAPK pathways. Biomed Pharmacother 94:1094–1100
Nozari S et al (2019) Potential anticancer effects of cell wall protein fractions from Lactobacillus paracasei on human intestinal Caco-2 cell line. Lett Appl Microbiol 69(3):148–154
Amirsaadat S et al (2017) Silibinin-loaded magnetic nanoparticles inhibit hTERT gene expression and proliferation of lung cancer cells. Artif Cells Nanomed Biotechnol 45(8):1649–1656
Sabahi S et al (2022) Production of functional ice cream fortified by immunoglobulin Y against Escherichia coli O157: H7 and Helicobacter pylori
Ozma MA et al (2022) Postbiotics as the key mediators of the gut microbiota-host interactions. Infez Med 30(2):180
Wang R et al (2022) Solvents effect on phenolics, iridoids, antioxidant activity, antibacterial activity, and pancreatic lipase inhibition activity of noni (Morinda citrifolia L.) fruit extract. Food Chem 377:131989
Molska M et al (2022) Analysis of phenolic compounds in buckwheat (Fagopyrum esculentum Moench) sprouts modified with probiotic yeast. Molecules 27(22):7773
Wang Z et al (2022) Fermentation of kiwifruit juice from two cultivars by probiotic bacteria: bioactive phenolics, antioxidant activities and flavor volatiles. Food Chem 373:131455
Doi K, Uetsuka K (2011) Mechanisms of mycotoxin-induced neurotoxicity through oxidative stress-associated pathways. Int J Mol Sci 12(8):5213–5237
Faustino M et al (2019) Agro-food byproducts as a new source of natural food additives. Molecules 24(6):1056
Amaretti A et al (2013) Antioxidant properties of potentially probiotic bacteria: in vitro and in vivo activities. Appl Microbiol Biotechnol 97:809–817
Guerrero-Encinas I et al (2021) Protective effect of Lacticaseibacillus casei CRL 431 postbiotics on mitochondrial function and oxidative status in rats with aflatoxin B 1–induced oxidative stress. Probiot Antimicro Prot 1–11
Wang Y et al (2017) Antioxidant properties of probiotic bacteria. Nutrients 9(5):521
Aguilar-Toalá J et al (2019) Modulatory effect of the intracellular content of Lactobacillus casei CRL 431 against the aflatoxin B 1-induced oxidative stress in rats. Probiot Antimicro Prot 11:470–477
Li H et al (2021) Study on the nutritional characteristics and antioxidant activity of dealcoholized sequentially fermented apple juice with Saccharomyces cerevisiae and Lactobacillus plantarum fermentation. Food Chem 363:130351
Chen Q et al (2021) Characterization and antioxidant activity of wheat bran polysaccharides modified by Saccharomyces cerevisiae and Bacillus subtilis fermentation. J Cereal Sci 97:103157
Yu Y-H et al (2020) Successful biosynthesis of natural antioxidant ergothioneine in Saccharomyces cerevisiae required only two genes from Grifola frondosa. Microb Cell Fact 19:1–10
Behbahani BA et al (2018) Oliveria decumbens essential oil: chemical compositions and antimicrobial activity against the growth of some clinical and standard strains causing infection. Microb Pathog 114:449–452
Falah F et al (2021) In vitro screening of phytochemicals, antioxidant, antimicrobial, and cytotoxic activity of Echinops setifer extract. Biocatal Agric Biotechnol 35:102102
Mehrnia MA et al (2021) Sclerorhachis platyrachis essential oil: antioxidant power, total phenolic and flavonoid content and its antimicrobial activity on some Gram-positive and Gram-negative bacteria “in vitro.” Journal of food science and technology (Iran) 18(112):189–198
Zanganeh H et al (2021) Evaluation of the chemical and antibacterial properties of Citrus paradise essential oil and its application in Lallemantia iberica seed mucilage edible coating to improve the physicochemical, microbiological and sensory properties of lamb during refrigerated storage. J Food Meas Charact 15(6):5556–5571
Unlu M et al (2010) Composition, antimicrobial activity and in vitro cytotoxicity of essential oil from Cinnamomum zeylanicum Blume (Lauraceae). Food Chem Toxicol 48(11):3274–3280
Ribeiro-Santos R et al (2017) Biological activities and major components determination in essential oils intended for a biodegradable food packaging. Ind Crops Prod 97:201–210
Pereira RP et al (2021) In vitro assessment of probiotic potential of Saccharomyces cerevisiae DABRP5 isolated from Bollo batter, a traditional Goan fermented food. Probiot Antimicro Prot 13:796–808
Abdollahzadeh E et al (2016) Prevalence and molecular characterization of Listeria spp. and Listeria monocytogenes isolated from fish, shrimp, and cooked ready-to-eat (RTE) aquatic products in Iran. LWT 73:205–21
Abdollahzadeh E et al (2016) Antimicrobial resistance of Listeria monocytogenes isolated from seafood and humans in Iran. Microb Pathog 100:70–74
Kim YJ et al (2021) Anti-biofilm effect of the cell-free supernatant of probiotic Saccharomyces cerevisiae against Listeria monocytogenes. Food Control 121:107667
Li R et al (2021) Listeria decontamination of chicken meat with beer brewed with bacteriocin producing Saccharomyces boulardii. LWT 152:112323
Koo OK, Kim SM, Kang SH (2015) Antimicrobial potential of Leuconostoc species against E. coli O157: H7 in ground meat. J Korean Soc Appl Biol Chem 58(6):831–838
Hamad G, Botros W, Hafez E (2017) Combination of probiotic filtrates as antibacterial agent against selected some pathogenic bacteria in milk and cheese. Int J Dairy Sci 12:368–376
Gálvez A et al (2007) Bacteriocin-based strategies for food biopreservation. Int J Food Microbiol 120(1–2):51–70
Sasikumar K et al (2017) An exopolysaccharide (EPS) from a Lactobacillus plantarum BR2 with potential benefits for making functional foods. Biores Technol 241:1152–1156
Bhat B, Bajaj BK (2018) Hypocholesterolemic and bioactive potential of exopolysaccharide from a probiotic Enterococcus faecium K1 isolated from kalarei. Biores Technol 254:264–267
Shamekhi S et al (2020) Apoptotic effect of Saccharomyces cerevisiae on human colon cancer SW480 cells by regulation of Akt/NF-ĸB signaling pathway. Probiot Antimicro Prot 12:311–319
Sima P, Richter J, Vetvicka V (2019) Glucans as new anticancer agents. Anticancer Res 39(7):3373–3378
Fortin O et al (2018) Cancer chemopreventive, antiproliferative, and superoxide anion scavenging properties of Kluyveromyces marxianus and Saccharomyces cerevisiae var. boulardii cell wall components. Nutr Cancer 70(1):83–96
Nami Y et al (2015) The prophylactic effect of probiotic Enterococcus lactis IW5 against different human cancer cells. Front Microbiol 6:1317
Plewka D et al (2018) Nuclear factor-kappa B as potential therapeutic target in human colon cancer. J Cancer Res Ther 14(3):516–520
Koerber MI et al (2016) NFκB-associated pathways in progression of chemoresistance to 5-fluorouracil in an in vitro model of colonic carcinoma. Anticancer Res 36(4):1631–1639
Davoudi Z et al (2014) Molecular target therapy of AKT and NF-kB signaling pathways and multidrug resistance by specific cell penetrating inhibitor peptides in HL-60 cells. Asian Pac J Cancer Prev 15(10):4353–4358
Brown K et al (1993) Mutual regulation of the transcriptional activator NF-kappa B and its inhibitor, I kappa B-alpha. Proc Natl Acad Sci 90(6):2532–2536
Karin M et al (2002) NF-κB in cancer: from innocent bystander to major culprit. Nat Rev Cancer 2(4):301–310
Altonsy MO, Andrews SC, Tuohy KM (2010) Differential induction of apoptosis in human colonic carcinoma cells (Caco-2) by Atopobium, and commensal, probiotic and enteropathogenic bacteria: mediation by the mitochondrial pathway. Int J Food Microbiol 137(2–3):190–203
Hu T et al (2016) Mechanisms of drug resistance in colon cancer and its therapeutic strategies. World J Gastroenterol 22(30):6876
Acknowledgements
The authors would like to express their thanks to the Toxicology Research Center, Medical Basic Sciences Research Institute of Ahvaz Jundishapur University of Medical Sciences for the financial support of this study.
Funding
This research was funded by the Toxicology Research Center, Medical Basic Sciences Research Institute of Ahvaz Jundishapur University of Medical Sciences, grant number TRC-0059.
Author information
Authors and Affiliations
Contributions
All of the authors contributed to the conception and design of the study and revised the manuscript. AA and SA conducted all the experiments and statistical analysis. AA, SS, and AYA drafted the first version of the manuscript. HH and SS reviewed the draft manuscript, and all the authors revised the final version of the manuscript.
Corresponding author
Ethics declarations
Ethics Approval and Consent to Participate
This study was approved by the ethics committee of the research and technology deputy of Ahvaz Jundishapur University of Medical Sciences (IR.AJUMS.REC.1400.609). All methods were carried out in accordance with relevant guidelines and regulations.
Consent for Publication
Not applicable.
Competing Interests
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Hosseini, H., Abbasi, A., Sabahi, S. et al. Assessing the Potential Biological Activities of Postbiotics Derived from Saccharomyces cerevisiae: An In Vitro Study. Probiotics & Antimicro. Prot. (2023). https://doi.org/10.1007/s12602-023-10117-y
Accepted:
Published:
DOI: https://doi.org/10.1007/s12602-023-10117-y