Abstract
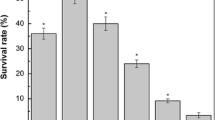
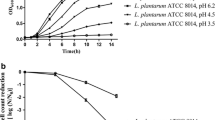
Lactobacillus casei strains have traditionally been recognized as probiotics and frequently used as adjunct culture in fermented dairy products where lactic acid stress is a frequently encountered environmental condition. We have investigated the effect of lactic acid stress on the cell membrane of L. casei Zhang [wild type (WT)] and its acid-resistant mutant Lbz-2. Both strains were grown under glucose-limiting conditions in chemostats; following challenge by low pH, the cell membrane stress responses were investigated. In response to acid stress, cell membrane fluidity decreased and its fatty acid composition changed to reduce the damage caused by lactic acid. Compared with the WT, the acid-resistant mutant exhibited numerous survival advantages, such as higher membrane fluidity, higher proportions of unsaturated fatty acids, and higher mean chain length. In addition, cell integrity analysis showed that the mutant maintained a more intact cellular structure and lower membrane permeability after environmental acidification. These results indicate that alteration in membrane fluidity, fatty acid distribution, and cell integrity are common mechanisms utilized by L. casei to withstand severe acidification and to reduce the deleterious effect of lactic acid on the cell membrane. This detailed comparison of cell membrane responses between the WT and mutant add to our knowledge of the acid stress adaptation and thus enable new strategies to be developed aimed at improving the industrial performance of this species under acid stress.








Similar content being viewed by others
References
Alakomi HL, Skytta E, Saarela M, Mattila-Sandholm T, Latva-Kala K, Helander IM (2000) Lactic acid permeabilizes Gram-negative bacteria by disrupting the outer membrane. Appl Environ Microb 66:2001–2005
Ansari S, Gupta P, Mahanty SK, Prasad R (1993) The uptake of amino acids by erg mutants of Candida albicans. Med Mycol 31:377–386
Aricha B, Fishov I, Cohen Z, Sikron N, Pesakhov S, Khozin-Goldberg I, Dagan R, Porat N (2004) Differences in membrane fluidity and fatty acid composition between phenotypic variants of Streptococcus pneumoniae. J Bacteriol 186:4638–4644
Barker C, Park S (2001) Sensitization of Listeria monocytogenes to low pH, organic acids, and osmotic stress by ethanol. Appl Environ Microb 67:1594–1600
Broadbent JR, Lin C (1999) Effect of heat shock or cold shock treatment on the resistance of Lactococcus lactis to freezing and lyophilization. Cryobiology 39:88–102
Budin-Verneuil A, Maguin E, Auffray Y, Ehrlich SD, Pichereau V (2005) Transcriptional analysis of the cyclopropane fatty acid synthase gene of Lactococcus lactis MG1363 at low pH. FEMS Microbiol Lett 250:189–194
Cao-Hoang L, Marechal PA, Lê-Thanh M, Gervais P, Waché Y (2008) Fluorescent probes to evaluate the physiological state and activity of microbial biocatalysts: a guide for prokaryotic and eukaryotic investigation. Biotechnol J 3:890–903
Chang YY, Cronan JE (1999) Membrane cyclopropane fatty acid content is a major factor in acid resistance of Escherichia coli. Mol Microbiol 33:249–259
Chu-Ky S, Tourdot-Marechal R, Marechal P, Guzzo J (2005) Combined cold, acid, ethanol shocks in Oenococcus oeni: effects on membrane fluidity and cell viability. BBA Biomembr 1717:118–124
De Angelis M, Gobbetti M (2004) Environmental stress responses in Lactobacillus: a review. Proteomics 4:106–122
Denich TJ, Beaudette LA, Lee H, Trevors JT (2003) Effect of selected environmental and physico-chemical factors on bacterial cytoplasmic membranes. J Microbiol Method 52:149–182
Fozo EM, Quivey RG (2004a) The fabM gene product of Streptococcus mutans is responsible for the synthesis of monounsaturated fatty acids and is necessary for survival at low pH. J Bacteriol 186:4152–4158
Fozo EM, Quivey RG (2004b) Shifts in the membrane fatty acid profile of Streptococcus mutans enhance survival in acidic environments. Appl Environ Microb 70:929–936
Fozo EM, Kajfasz JK, Quivey RG (2004) Low pH-induced membrane fatty acid alterations in oral bacteria. FEMS Microbiol Lett 238:291–295
Guerzoni ME, Lanciotti R, Cocconcelli PS (2001) Alteration in cellular fatty acid composition as a response to salt, acid, oxidative and thermal stresses in Lactobacillus helveticus. Microbiol 147:2255–2264
Guillot A, Obis D, Mistou MY (2000) Fatty acid membrane composition and activation of glycine-betaine transport in Lactococcus lactis subjected to osmotic stress. Int J Food Microbiol 55:47–51
Kleerebezem M, Vaughan EE (2009) Probiotic and gut lactobacilli and bifidobacteria: molecular approaches to study diversity and activity. Annu Rev Microbiol 63:269–290
Lehrer RI, Barton A, Ganz T (1988) Concurrent assessment of inner and outer membrane permeabilization and bacteriolysis in E. coli by multiple-wavelength spectrophotometry. J Immunol Methods 108:153–158
Marco ML, Pavan S, Kleerebezem M (2006) Towards understanding molecular modes of probiotic action. Curr Opin Biotechnol 17:204–210
Mykytczuk NCS, Trevors JT, Leduc LG, Ferroni GD (2007) Fluorescence polarization in studies of bacterial cytoplasmic membrane fluidity under environmental stress. Prog Biophys Mol Biol 95:60–82
Parvez S, Malik KA, Kang SA, Kim HY (2006) Probiotics and their fermented food products are beneficial for health. J Appl Microbiol 100:1171–1185
Rodriguez-Vargas S, Sanchez-Garcia A, Martinez-Rivas JM, Prieto JA, Randez-Gil F (2007) Fluidization of membrane lipids enhances the tolerance of Saccharomyces cerevisiae to freezing and salt stress. Appl Environ Microb 73:110–116
Russell NJ (1984) Mechanisms of thermal adaptation in bacteria: blueprints for survival. Trends Biochem Sci 9:108–112
Sikkema J, De Bont J, Poolman B (1995) Mechanisms of membrane toxicity of hydrocarbons. Microbiol Mol Biol Rev 59:201–222
Streit F, Delettre J, Corrieu G, Beal C (2008) Acid adaptation of Lactobacillus delbrueckii subsp. bulgaricus induces physiological responses at membrane and cytosolic levels that improves cryotolerance. J Appl Microbiol 105:1071–1080
Walter A, Gutknecht J (1984) Monocarboxylic acid permeation through lipid bilayer membranes. J Membr Biol 77:255–264
Wang Y, Corrieu G, Béal C (2005) Fermentation pH and temperature influence the cryotolerance of Lactobacillus acidophilus RD758. J Dairy Sci 88:21–29
Warnecke T, Gill RT (2005) Organic acid toxicity, tolerance, and production in Escherichia coli biorefining applications. Microb Cell Fact 4:25–32
Zaritsky A, Parola AH, Abdah M, Masalha H (1985) Homeoviscous adaptation, growth rate, and morphogenesis in bacteria. Biophys J 48:337–339
Zhang J, Du G, Zhang Y, Liao X, Wang M, Li Y, Chen J (2010) Glutathione protects Lactobacillus sanfranciscensis against freeze-thawing, freeze-drying, and cold treatment. Appl Environ Microb 76:2989–2996
Zhang YM, Rock CO (2008) Membrane lipid homeostasis in bacteria. Nat Rev Microbiol 6:222–233
Zhu Y, Zhang Y, Li Y (2009) Understanding the industrial application potential of lactic acid bacteria through genomics. Appl Microbiol Biotechnol 83:597–610
Acknowledgments
This project was financially supported by the Key Program of National Natural Science Foundation of China (No. 20836003), the National Natural Science Foundation of China (No. 30900013), and the Major State Basic Research Development Program of China (973 Program, No. 2007CB714306).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Chongde Wu and Juan Zhang contributed equally to this work and are co-first authors.
Rights and permissions
About this article
Cite this article
Wu, C., Zhang, J., Wang, M. et al. Lactobacillus casei combats acid stress by maintaining cell membrane functionality. J Ind Microbiol Biotechnol 39, 1031–1039 (2012). https://doi.org/10.1007/s10295-012-1104-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10295-012-1104-2