Abstract
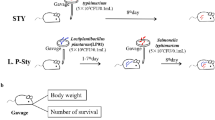
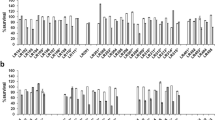
The aim of this study was to evaluate the antibacterial potential of lactic acid bacteria (Weissella confuse, Pediococcus acidilactici, and Ligilactobacillus equi) isolated from healthy equine in Wuhan against Salmonella Typhimurium CVCC542-induced mice model on intestinal microflora. In previous studies, these isolated strains showed good probiotic potentials in vitro. In this study, fifty healthy mice were randomly divided into five groups, the blank control group, the control group, the Pediococcus acidilactici group (1 × 108 CFU/day), the Ligilactobacillus equi group (1 × 108 CFU/day), and the Weissella confuse group (1 × 108 CFU/day). The body weight in control group and Weissella confuse group showed significant decreased (P < 0.05, P < 0.01), while Pediococcus acidilactici group and Ligilactobacillus equi group showed good recovering after treatments. The lowest diarrhea rate was shown in Ligilactobacillus equi group after treatment. In histopathology, Ligilactobacillus equi group showed the least structural damage in duodenum, and all probiotic treatment groups showed less damage in cecum. The sequence data and optical transform unit showed that Pediococcus acidilactici group and Ligilactobacillus equi group had higher number than control group, while the diversity data showed that the control group and Weissella confuse group had lower diversity in cecum. Microbial community analysis showed increased abundance of Firmicutes, Bacteroidetes, uncultured_bacterium_f_Muribaculaceae, and Lactobacillus in treatment groups, while potential microbes that can induce intestinal diseases such as Verrucomicrobia, Akkermansia, and Lachnospiraceae_NK4A136_group decreased in the treatment groups. In conclusion, lactic acid bacteria isolated from the healthy horses could alleviate the infection of Salmonella and regulate intestinal flora.








Similar content being viewed by others
Availability of Data and Materials
Data are available on reasonable request to the authors.
References
Kirk MD, Pires SM, Black RE et al (2015) World Health Organization estimates of the global and regional disease burden of 22 foodborne bacterial, protozoal, and viral diseases, 2010: a data synthesis. PLoS Med 12:e1001921. https://doi.org/10.1371/journal.pmed.1001921
Kasimoglu Dogru A, Ayaz ND, Gencay YE (2010) Serotype identification and antimicrobial resistance profiles of Salmonella spp. isolated from chicken carcasses. Trop Anim Health Prod 42:893–897. https://doi.org/10.1007/s11250-009-9504-7
Scallan E, Hoekstra RM, Mahon BE, Jones TF, Griffin PM (2015) An assessment of the human health impact of seven leading foodborne pathogens in the United States using disability adjusted life years. Epidemiol Infect 143:2795–2804. https://doi.org/10.1017/S0950268814003185
Majowicz SE, Musto J, Scallan E et al (2010) The global burden of nontyphoidal Salmonella gastroenteritis. Clin Infect Dis 50:882–889. https://doi.org/10.1086/650733
Liu H, Whitehouse CA, Li B (2018) Presence and persistence of Salmonella in water: the impact on microbial quality of water and food safety. Front Public Health 6:159. https://doi.org/10.3389/fpubh.2018.00159
He H, Genovese KJ, Swaggerty CL, Nisbet DJ, Kogut MH (2012) A comparative study on invasion, survival, modulation of oxidative burst, and nitric oxide responses of macrophages (HD11), and systemic infection in chickens by prevalent poultry Salmonella serovars. Foodborne Pathog Dis 9:1104–1110. https://doi.org/10.1089/fpd.2012.1233
Jackson BR, Griffin PM, Cole D, Walsh KA, Chai SJ (2013) Outbreak-associated Salmonella enterica serotypes and food commodities, United States, 1998–2008. Emerg Infect Dis 19:1239–1244. https://doi.org/10.3201/eid1908.121511
Ford L, Moffatt CRM, Fearnley E et al (2018) The epidemiology of Salmonella enterica outbreaks in Australia, 2001–2016. Frontiers in Sustainable Food Systems 2:86. https://doi.org/10.3389/fsufs.2018.00086
Bouchrif B, Paglietti B, Murgia M, Piana A, Cohen N, Ennaji MM, Rubino S, Timinouni M (2009) Prevalence and antibiotic-resistance of Salmonella isolated from food in Morocco. J Infect Dev Ctries 3:35–40. https://doi.org/10.3855/jidc.103
Threlfall EJ, Ward LR, Frost JA, Willshaw GA (2000) The emergence and spread of antibiotic resistance in food-borne bacteria. Int J Food Microbiol 62:1–5. https://doi.org/10.1016/S0168-1605(00)00351-2
Meakins S, Fisher IS, Berghold C et al (2008) Antimicrobial drug resistance in human nontyphoidal Salmonella isolates in Europe 2000–2004: a report from the enter-net international surveillance network. Microb Drug Resist 14:31–35. https://doi.org/10.1089/mdr.2008.0777
Kariuki S, Gordon MA, Feasey N, Parry CM (2015) Antimicrobial resistance and management of invasive Salmonella disease. Vaccine 33(Suppl 3):C21–C29. https://doi.org/10.1016/j.vaccine.2015.03.102
Antunes LC, Han J, Ferreira RB, Lolić P, Borchers CH, Finlay BB (2011) Effect of antibiotic treatment on the intestinal metabolome. Antimicrob Agents Chemother 55:1494–1503. https://doi.org/10.1128/AAC.01664-10
Conte MP, Schippa S, Zamboni I, Penta M, Chiarini F, Seganti L, Osborn J, Falconieri P, Borrelli O, Cucchiara S (2006) Gut-associated bacterial microbiota in paediatric patients with inflammatory bowel disease. Gut 55:1760–1767. https://doi.org/10.1136/gut.2005.078824
Modi SR, Collins JJ, Relman DA (2014) Antibiotics and the gut microbiota. J Clin Invest 124:4212–4218. https://doi.org/10.1172/JCI72333
Lupp C, Robertson ML, Wickham ME, Sekirov I, Champion OL, Gaynor EC, Finlay BB (2007) Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2:119–129. https://doi.org/10.1016/j.chom.2007.06.010
Sekirov I, Tam NM, Jogova M, Robertson ML, Li Y, Lupp C, Finlay BB (2008) Antibiotic-induced perturbations of the intestinal microbiota alter host susceptibility to enteric infection. Infect Immun 76:4726–4736. https://doi.org/10.1128/IAI.00319-08
Bergogne-Bérézin E (2000) Treatment and prevention of antibiotic associated diarrhea. Int J Antimicrob Agents 16:521–526. https://doi.org/10.1016/s0924-8579(00)00293-4
Kaltenbach G, Heitz D (2004) Antibiotic-associated diarrhea in the elderly. Rev Med Interne 25:46–53. https://doi.org/10.1016/j.revmed.2003.10.002
De Briyne N, Atkinson J, Pokludová L, Borriello SP (2014) Antibiotics used most commonly to treat animals in Europe. Vet Rec 175:325. https://doi.org/10.1136/vr.102462
Zhang H, Rehman MU, Li K et al (2016) Antimicrobial resistance of Escherichia coli isolated from Tibetan piglets suffering from white score diarrhea. Pak Vet J 37:43–46
Li A, Wang Y, Pei L, Mehmood K, Li K, Qamar H, Iqbal M, Waqas M, Liu J, Li J (2019) Influence of dietary supplementation with Bacillus velezensis on intestinal microbial diversity of mice. Microb Pathog 136:103671. https://doi.org/10.1016/j.micpath.2019.103671
Allen HK, Levine UY, Looft T, Bandrick M, Casey TA (2013) Treatment, promotion, commotion: antibiotic alternatives in food-producing animals. Trends Microbiol 21:114–119. https://doi.org/10.1016/j.tim.2012.11.001
Asahara T, Shimizu K, Takada T, Kado S, Yuki N, Morotomi M, Tanaka R, Nomoto K (2011) Protective effect of Lactobacillus casei strain Shirota against lethal infection with multi-drug resistant Salmonella enterica serovar Typhimurium DT104 in mice. J Appl Microbiol 110:163–173. https://doi.org/10.1111/j.1365-2672.2010.04884.x
Deriu E, Liu JZ, Pezeshki M, Edwards RA, Ochoa RJ, Contreras H, Libby SJ, Fang FC, Raffatellu M (2013) Probiotic bacteria reduce Salmonella Typhimurium intestinal colonization by competing for iron. Cell Host Microbe 14:26–37. https://doi.org/10.1016/j.chom.2013.06.007
Khan S, Chousalkar KK (2020) Salmonella Typhimurium infection disrupts but continuous feeding of Bacillus based probiotic restores gut microbiota in infected hens. J Anim Sci Biotechnol 11:29. https://doi.org/10.1186/s40104-020-0433-7
Naik AK, Pandey U, Mukherjee R, Mukhopadhyay S, Chakraborty S, Ghosh A, Aich P (2019) Lactobacillus rhamnosus GG reverses mortality of neonatal mice against Salmonella challenge. Toxicol Res (Camb) 8:361–372. https://doi.org/10.1039/c9tx00006b
Burkholder KM, Fletcher DH, Gileau L, Kandolo A (2019) Lactic acid bacteria decrease Salmonella enterica Javiana virulence and modulate host inflammation during infection of an intestinal epithelial cell line. Pathog Dis 77:ftz025. https://doi.org/10.1093/femspd/ftz025
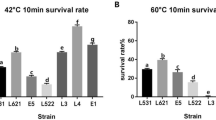
Pei L, Yang H, Qin S, Yan Z, Zhang H, Lan Y, Li A, Iqbal M, Shen Y (2021) Isolation and evaluation of probiotic potential of lactic acid strains from healthy equines for potential use in Salmonella infection. J Equine Vet Sci 96:103312. https://doi.org/10.1016/j.jevs.2020.103312
Bolick DT, Kolling GL, Moore JH 2nd et al (2014) Zinc deficiency alters host response and pathogen virulence in a mouse model of enteroaggregative Escherichia coli-induced diarrhea. Gut Microbes 5:618–627. https://doi.org/10.4161/19490976.2014.969642
Yang JY, Lee SN, Chang SY, Ko HJ, Ryu S, Kweon MN (2014) A mouse model of shigellosis by intraperitoneal infection. J Infect Dis 209:203–215. https://doi.org/10.1093/infdis/jit399
Costello EK, Lauber CL, Hamady M, Fierer N, Gordon JI, Knight R (2009) Bacterial community variation in human body habitats across space and time. Science 326:1694–1697. https://doi.org/10.1126/science.1177486
Fujimura KE, Slusher NA, Cabana MD, Lynch SV (2010) Role of the gut microbiota in defining human health. Expert Rev Anti Infect Ther 8:435–454. https://doi.org/10.1586/eri.10.14
Arumugam M, Raes J, Pelletier E et al (2011) Enterotypes of the human gut microbiome. Nature 473:174–180. https://doi.org/10.1038/nature09944
Endt K, Stecher B, Chaffron S et al (2010) The microbiota mediates pathogen clearance from the gut lumen after non-typhoidal Salmonella diarrhea. PLoS Pathog 6:e1001097. https://doi.org/10.1371/journal.ppat.1001097
Li A, Wang Y, Li Z, Qamar H, Mehmood K, Zhang L, Liu J, Zhang H, Li J (2019) Probiotics isolated from yaks improves the growth performance, antioxidant activity, and cytokines related to immunity and inflammation in mice. Microb Cell Fact 18:112. https://doi.org/10.1186/s12934-019-1161-6
Zeng Y, Zeng D, Zhang Y, Ni X, Tang Y, Zhu H, Wang H, Yin Z, Pan K, Jing B (2015) Characterization of the cellulolytic bacteria communities along the gastrointestinal tract of Chinese Mongolian sheep by using PCR-DGGE and real-time PCR analysis. World J Microbiol Biotechnol 31:1103–1113. https://doi.org/10.1007/s11274-015-1860-z
Zhao W, Wang Y, Liu S, Huang J, Zhai Z, He C, Ding J, Wang J, Wang H, Fan W, Zhao J, Meng H (2015) The dynamic distribution of porcine microbiota across different ages and gastrointestinal tract segments. PLoS One 10:e0117441. https://doi.org/10.1371/journal.pone.0117441
O’Hara AM, Shanahan F (2006) The gut flora as a forgotten organ. EMBO Rep 7:688–693. https://doi.org/10.1038/sj.embor.7400731
Yan W, Sun C, Yuan J, Yang N (2017) Gut metagenomic analysis reveals prominent roles of Lactobacillus and cecal microbiota in chicken feed efficiency. Sci Rep 7:45308. https://doi.org/10.1038/srep45308
Ley RE, Hamady M, Lozupone C et al (2008) Evolution of mammals and their gut microbes. Science 320:1647–1651. https://doi.org/10.1126/science.1155725
Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, Krakoff J (2011) Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr 94:58–65. https://doi.org/10.3945/ajcn.110.010132
Turnbaugh PJ, Bäckhed F, Fulton L, Gordon JI (2008) Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe 3:213–223. https://doi.org/10.1016/j.chom.2008.02.015
De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, Collini S, Pieraccini G, Lionetti P (2010) Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A 107:14691–14696. https://doi.org/10.1073/pnas.1005963107
Zeyda M, Stulnig TM (2009) Obesity, inflammation, and insulin resistance–a mini-review. Gerontology 55:379–386. https://doi.org/10.1159/000212758
Manabe I (2011) Chronic inflammation links cardiovascular, metabolic and renal diseases. Circ J 75:2739–2748. https://doi.org/10.1253/circj.cj-11-1184
Okwan-Duodu D, Umpierrez GE, Brawley OW, Diaz R (2013) Obesity-driven inflammation and cancer risk: role of myeloid derived suppressor cells and alternately activated macrophages. Am J Cancer Res 3:21–33
Jin H, Zhang C (2020) High Fat High Calories Diet (HFD) increase gut susceptibility to carcinogens by altering the gut microbial community. J Cancer 11:4091–4098. https://doi.org/10.7150/jca.43561
Hui H, Wu Y, Zheng T, Zhou S, Tan Z (2020) Bacterial characteristics in intestinal contents of antibiotic-associated diarrhea mice treated with qiweibaizhu powder. Med Sci Monit 26:e921771. https://doi.org/10.12659/MSM.921771
Collado MC, Derrien M, Isolauri E, de Vos WM, Salminen S (2007) Intestinal integrity and Akkermansia muciniphila, a mucin-degrading member of the intestinal microbiota present in infants, adults, and the elderly. Appl Environ Microbiol 73:7767–7770. https://doi.org/10.1128/AEM.01477-07
Campieri M, Gionchetti P (2001) Bacteria as the cause of ulcerative colitis. Gut 48:132–135. https://doi.org/10.1136/gut.48.1.132
Zheng J, Yuan X, Cheng G, Jiao S, Feng C, Zhao X, Yin H, Du Y, Liu H (2018) Chitosan oligosaccharides improve the disturbance in glucose metabolism and reverse the dysbiosis of gut microbiota in diabetic mice. Carbohydr Polym 190:77–86. https://doi.org/10.1016/j.carbpol.2018.02.058
Chassaing B, Gewirtz AT (2014) Gut microbiota, low-grade inflammation, and metabolic syndrome. Toxicol Pathol 42:49–53. https://doi.org/10.1177/0192623313508481
Cui HX, Zhang LS, Luo Y, Yuan K, Huang ZY, Guo Y (2019) A purified anthraquinone-glycoside preparation from rhubarb ameliorates type 2 diabetes mellitus by modulating the gut microbiota and reducing inflammation. Front Microbiol 10:1423. https://doi.org/10.3389/fmicb.2019.01423
Salzman NH, de Jong H, Paterson Y, Harmsen HJM, Welling GW, Bos NA (2002) Analysis of 16S libraries of mouse gastrointestinal microflora reveals a large new group of mouse intestinal bacteria. Microbiology (Reading) 148:3651–3660. https://doi.org/10.1099/00221287-148-11-3651
Lagkouvardos I, Pukall R, Abt B et al (2016) The mouse intestinal Bacterial Collection (miBC) provides host-specific insight into cultured diversity and functional potential of the gut microbiota. Nat Microbiol 1:16131. https://doi.org/10.1038/nmicrobiol.2016.131
Seedorf H, Griffin NW, Ridaura VK et al (2014) Bacteria from diverse habitats colonize and compete in the mouse gut. Cell 159:253–266. https://doi.org/10.1016/j.cell.2014.09.008
Ormerod KL, Wood DL, Lachner N et al (2016) Genomic characterization of the uncultured Bacteroidales family S24–7 inhabiting the guts of homeothermic animals. Microbiome 4:36. https://doi.org/10.1186/s40168-016-0181-2
Lagkouvardos I, Lesker TR, Hitch TCA et al (2019) Sequence and cultivation study of Muribaculaceae reveals novel species, host preference, and functional potential of this yet undescribed family. Microbiome 7:28. https://doi.org/10.1186/s40168-019-0637-2
Yıldız GG, Öztürk M, Aslım B (2011) Identification of Lactobacillus strains from breast-fed infant and investigation of their cholesterol-reducing effects. World J Microbiol Biotechnol 27:2397–2406. https://doi.org/10.1007/s11274-011-0710-x
Walker WA (2000) Role of nutrients and bacterial colonization in the development of intestinal host defense. J Pediatr Gastroenterol Nutr 30(Suppl 2):S2–S7
Vesterlund S, Karp M, Salminen S, Ouwehand AC (2006) Staphylococcus aureus adheres to human intestinal mucus but can be displaced by certain lactic acid bacteria. Microbiology (Reading) 152:1819–1826. https://doi.org/10.1099/mic.0.28522-0
Collado MC, Meriluoto J, Salminen S (2007) Role of commercial probiotic strains against human pathogen adhesion to intestinal mucus. Lett Appl Microbiol 45:454–460. https://doi.org/10.1111/j.1472-765X.2007.02212.x
Isolauri E, Sütas Y, Kankaanpää P, Arvilommi H, Salminen S (2001) Probiotics: effects on immunity. Am J Clin Nutr 73:444S-450S. https://doi.org/10.1093/ajcn/73.2.444s
Chen CY, Tsen HY, Lin CL, Yu B, Chen CS (2012) Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poult Sci 91:2139–2147. https://doi.org/10.3382/ps.2012-02237
Yan X, Feng B, Li P, Tang Z, Wang L (2016) Microflora disturbance during progression of glucose intolerance and effect of sitagliptin: an animal study. J Diabetes Res 2016:2093171. https://doi.org/10.1155/2016/2093171
Zhang Q, Yu H, Xiao X, Hu L, Xin F, Yu X (2018) Inulin-type fructan improves diabetic phenotype and gut microbiota profiles in rats. PeerJ 6:e4446. https://doi.org/10.7717/peerj.4446
Funding
The study was supported by the Fundamental Research Funds for the Central Universities, China (2662017QD015).
Author information
Authors and Affiliations
Contributions
LL Pei and YQ Shen proposed and designed the research idea; JJ Liu and ZH Huang contributed sample collection and analysis tools; LL Pei wrote the manuscript; M Iqbal checked the manuscript; YQ Shen checked the manuscript and approved the work done in the study.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Pei, L., Liu, J., Huang, Z. et al. Effects of Lactic Acid Bacteria Isolated from Equine on Salmonella-Infected Gut Mouse Model. Probiotics & Antimicro. Prot. 15, 469–478 (2023). https://doi.org/10.1007/s12602-021-09841-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-021-09841-0