Abstract
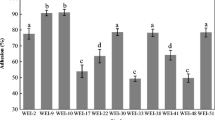
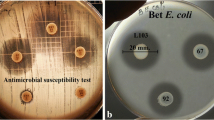
Three hundred and sixty presumptive lactic acid bacteria (LAB) isolated from pregnant sows, newborn, suckling, and weaned piglets were preliminarily screened for anti-Salmonella activity. Fifty-eight isolates consisting of Lactobacillus reuteri (n = 32), Lactobacillus salivarius (n = 10), Lactobacillus mucosae (n = 8), Lactobacillus johnsonii (n = 5), and Lactobacillus crispatus (n = 3) were selected and further characterized for probiotic properties including production of antimicrobial substances, acid and bile tolerance, and cell adherence to Caco-2 cells. Eight isolates including Lact. johnsonii LJ202 and Lact. reuteri LR108 were identified as potential probiotics. LJ202 was selected for further use in co-culture studies of two-bacterial and multiple-bacterial species to examine its inhibitory activity against Salmonella enterica serovar Enteritidis DMST7106 (SE7106). Co-culture of LJ202 and SE7106 showed that LJ202 could completely inhibit the growth of SE7106 in 10 h of co-culture. In co-culture of multiple-bacterial species, culturable fecal bacteria from pig feces were used as representative of multiple-bacterial species. The study was performed to examine whether interactions among multiple-bacterial species would influence antagonistic activity of LJ202 against SE7106 and fecal coliform bacteria. Co-culture of SE7106 with different combinations of fecal bacteria and probiotic (LJ202 and LR108) or non-probiotic (Lact. mucosae LM303) strains revealed that the growth of SE7106 was completely inhibited either in the presence or in the absence of probiotic strains. Intriguingly, LJ202 exhibited notable inhibitory activity against fecal coliform bacteria while LR108 did not. Taken together, the results of co-culture studies suggested that LJ202 is a good probiotic candidate for further study its inhibitory effects against pathogen infections in pigs.




Similar content being viewed by others
References
Kreuzer S, Janczyk P, Assmus J, Schmidt MF, Brockmann GA, Nockler K (2012) No beneficial effects evident for Enterococcus faecium NCIMB 10415 in weaned pigs infected with Salmonella enterica serovar Typhimurium DT104. Appl Environ Microbiol 78:4816–4825
Mathew AG, Cissell R, Liamthong S (2007) Antibiotic resistance in bacteria associated with food animals: a United States perspective of livestock production. Foodborne Pathog Dis 4:115–133
Regulation 1831/2003/EC. Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Official Journal of the European Union 268
FAO/WHO (2001) Health and nutritional properties of probiotics in food including powder milk with live lactic acid bacteria. FAO/WHO, Rome
Veizaj-Delia E, Piu T, Lekaj P, Tafaj M (2010) Using combined probiotic to improve growth performance of weaned piglets on extensive farm conditions. Livest Sci 134:249–251
Wang J, Ji HF, Hou CL, Wang SX, Zhang DY, Liu H, Shan DC, Wang YM (2014) Effects of Lactobacillus johnsonii X54 supplementation on reproductive performance, gut environment, and blood biochemical and immunological index in lactating sows. Livest Sci 164:96–101
Lähteinen T, Lindholm A, Rinttilä T, Junnikkala S, Kant R, Pietilä TE, Levonen K, von Ossowski I, Solano-Aguilar G, Jakava-Viljanen M, Palva A (2014) Effect of Lactobacillus brevis ATCC 8287 as a feeding supplement on the performance and immune function of piglets. Vet Immunol Immunopathol 158:14–25
Lähteinen T, Rinttilä T, Koort JMK, Kant R, Levonen K, Jakava-Viljanen M, Björkroth J, Palva A (2015) Effects of a multispecies Lactobacillus formation as a feeding supplement on the performance and immune function of piglets. Livest Sci 180:164–171
Higgins JP, Higgins SE, Wolfenden AD, Henderson SN, Torres-Rodiguez A, Vicente JL, Hargis BM, Tellez G (2010) Effect of lactic acid bacteria probiotic culture treatment timing on Salmonella Enteritidis in neonatal broilers. Poult Sci 89:243–247
Chen CY, Tsen HY, Lin CL, Yu B, Chen CS (2012) Oral administration of a combination of select lactic acid bacteria strains to reduce the Salmonella invasion and inflammation of broiler chicks. Poult Sci 91:2139–2147
Feng J, Wang L, Zhou L, Yang X, Zhao X (2016) Using in vitro immunomodulatory properties of lactic acid bacteria for selection of probiotics against Salmonella infection in broiler chicks. PLoS One 11(1):e0147630. doi:10.1371/journal. pone.0147630
Tsai CC, Hsih HY, Chiu HH, Lai YY, Liu JH, Yu B, Tsen HY (2005) Antagonistic activity against Salmonella infection in vitro and in vivo for two Lactobacillus strains from swine and poultry. Int J Food Microbiol 102:185–194
Lin CK, Tsai HC, Lin PP, Tsen HY, Tsai CC (2008) Lactobacillus acidophilus LAP5 able to inhibit the Salmonella Choleraesuis invasion to the human Caco-2 epithelial cell. Anaerobe 14:251–255
Casey PG, Gardiner GE, Casey G, Bradshaw B, Lawlor PG, Lynch PB, Leonard FC, Stanton C, Ross RP, Fitzgerald GF, Hill C (2007) A five-strain probiotic combination reduced pathogen shedding and alleviates disease signs in pigs challenged with Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 73:1858–1863
Barbosa TM, Serra CR, La Ragione RM, Woodward MJ, Henriques AO (2005) Screening for Bacillus isolates in the broiler gastrointestinal tract. Appl Environ Microbiol 71:968–978
Schillinger U, Lücke FK (1989) Antibacterial activity of Lactobacillus sake isolated from meat. Appl Environ Microbiol 55:1901–1906
Chauvière G, Coconnier MH, Kernéis S, Fourniat J, Servin AL (1992) Adhesion of human Lactobacillus acidophilus strain LB to human enterocyte-like Caco-2 cells. J Gen Microbiol 138:1689–1696
WigneswaranV, AmadorCI, JelsbakL, SternbergC, JelsbakL (2016) Utilization and control of ecological interactions in polymicrobial infections and community-based microbial cell factories [version 1; referees:3 approved]. F1000 Research. doi:10.12688/f1000research.7878.1
Leser TD, Amenuvor JZ, Jensen TK, Lindecrona RH, Boye M, Møller K (2002) Culture-independent analysis of gut bacteria: the pig gastrointestinal tract microbiota revisited. Appl Environ Microbiol 68:673–690
Yang F, Wang A, Zeng X, Hou C, Liu H, Qiao S (2015) Lactobacillus reuteri I5007 modulates tight junction protein expression in IPEC-J2 cells with LPS stimulation and in newborn piglets under normal conditions. BMC Microbiol 15:32. doi:10.1186/s12866-015-0372-1
Zhang J, Deng J, Wang Z, Che C, Li YF, Yang Q (2011) Modulatory effects of Lactobacillus salivarius on intestinal mucosal immunity of piglets. Curr Microbiol 62:1623–1631
Rubin HE, Nerad T, Vaughan F (1982) Lactate acid inhibition of Salmonella Typhimurium in yogurt. J Dairy Sci 65:197–203
Fayol-Messaoudi D, Berger CN, Coconnier-Polter MH, Liévin-LeMoal V, Servin AL (2005) pH-, lactic acid-, and non-lactic acid-dependent activities of probiotic Lactobacillus against Salmonella enterica serovar Typhimurium. Appl Environ Microbiol 71:6008–6013
Nouaille S, Even S, Charlier C, Le Loir Y, Cocaign-Bousquet M, Loubière P (2009) Transcriptome response of Lactococcus lactis in mixed culture with Staphylococcus aureus. Appl Environ Microbiol 75:4473–4482
Savage DC (1977) Microbiology of the gastrointestinal tract. Annu Rev Microbiol 31:107–133
De Vuyst L, Leroy F (2007) Bacteriocins from lactic acid bacteria: production, purification, and food applications. J Mol Microbiol Biotechnol 13:194–199
Melin L, Mattsson S, Katouli M, Wallgren P (2004) Development of post-weaning diarrhoea in piglets. Relation to presence of Escherichia coli strains and rotavirus. J Vet Med B Infect Dis Vet Public Health 51:12–22
Shu Q, Qu F, Gill HS (2001) Probiotic treatment using Bifidobacterium lactis HN019 reduces weanling diarrhoea associated with rotavirus and Escherichia coli infection in a piglet model. J Pediatr Gastroenterol Nutr 33:171–177
Huang CH, Qiao SY, Li DF, Piao XS, Ren JP (2004) Effects of lactobacilli on the performance, diarrhea incidence, VFA concentration and gastrointestinal microbial flora of weaning pigs. Asian Australas J Anim Sci 17:401–409
Mallo JJ, Rioperez J, Honrubia P (2010) The addition of Enterococcus faecium to diets improves piglet’s intestinal microbiota and performance. Livest Sci 133:176–178
Liu H, Ji HF, Zhang DY, Wang SX, Wang J, Shan DC, Wang YM (2015) Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest Sci 178:251–254
Peng Q, Zeng XF, Zhu JL, Wang S, Liu XT, Hou CL, Thacker PA, Qiao SY (2016) Effects of dietary Lactobacillus plantarum B1 on growth performance, intestinal microbiota, and short chain fatty acid profiles in broiler chickens. Poult Sci 95:893–900
Wang S, Peng Q, Jia HM, Zeng XF, Zhu JL, Hou CL, Liu XT, Yang FJ, Qiao SY (2017) Prevention of Escherichia coliinfection in broiler chickens with Lactobacillus plantarum B1. Poult Sci. doi:10.3382/ps/pex061
Haberbeck LU, Oliveira RC, Vivijis B, Wenseleers T, Aertsen A, Michiels C, Geeraerd AH (2015) Viability in growth/no growth boundaries of 188 different Escherichia colistrains reveals that approximately 75% have a higher growth probability under low pH conditions than E. coliO157:H7 strain ATCC 43888. Food Microbiol 45:222–230
Abee T, Klaenhammer TR, Letellier L (1994) Kinetic studies of the action of lactacin F, a bacteriocin produced by Lactobacillus johnsonii that forms poration complexs in the cytoplasmic membrane. Appl Environ Microbiol 60:1006–1013
Drissi F, Merhej V, Blanc-Tailleur C, Raoult D (2015) Draft genome sequence of the Lactobacillus mucosae Marseille. Genome Announc 3(4):e00841–e00815. doi:10.1128/genomeA.00841-15
Ramsey MM, Rumbaugh KP, Whiteley M (2011) Metabolite cross-feeding enhances virulence in a model polymicrobial infection. PLoS Pathog 7(3):e1002012. doi:10.1371/journaLact.ppat.1002012
Chubiz LM, Granger BR, Segrè D, Harcombe WR (2015) Species interactions differ in their genetic robustness. Front Microbiol 6:271. doi:10.3389/fmicb.2015.00271
Chanter N (1997) Streptococci and enterococci as animal pathogens. J Appl Microbiol Symp Suppl 83:100S–109S
Acknowledgements
This study was funded by the National Center for Genetic Engineering and Biotechnology (BIOTEC), Thailand (grant number P-14-50177). We thank Ms. Sukitaya Veeranondha for her assistance in the culture of Caco-2 cells.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Abhisingha, M., Dumnil, J. & Pitaksutheepong, C. Selection of Potential Probiotic Lactobacillus with Inhibitory Activity Against Salmonella and Fecal Coliform Bacteria. Probiotics & Antimicro. Prot. 10, 218–227 (2018). https://doi.org/10.1007/s12602-017-9304-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-017-9304-8