Abstract
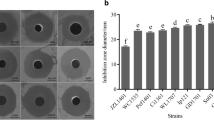
Use of probiotics as the biocontrol agent for disease prevention in aquaculture is gaining importance as an alternative to the indiscriminate use of antibiotics and other chemotherapeutics. In view of this trend, the probiotic properties of a potent antagonistic bacterium, Pseudomonas aeruginosa FARP72, was characterized in terms of safety, antagonistic activities, in vitro immunomodulation, and in vivo disease resistance. Immunomodulatory activity was ascertained by measuring the production of intracellular superoxide anion, nitric oxide, total leukocyte peroxidase content, and the leukocyte proliferation in head kidney leukocytes. The bacterium isolated from the skin mucus of freshwater catfish Clarias batrachus was harmless to Labeo rohita. It showed inhibitory activity against Aeromonas caviae, A. hydrophila, Edwardsiella tarda, Pseudomonas putida, and Streptococcus agalactiae as revealed by cross and parallel streaking methods. Significantly higher superoxide anion and nitric oxide production, peroxidase content, and proliferative responses of leucocytes delineated the strains’ ability to interact with immune cells to activate the immune system in vitro. Significant growth inhibition of A. hydrophila from 1.55 × 105 CFU/mL was observed when co-cultured with P. aeruginosa FARP72 in phosphate-buffered saline (PBS) at levels ranging from 2.61 × 107 to 2.61 × 109 CFU/mL in 10 days. P. aeruginosa FARP72 increased the survival rate of rohu fingerlings against pathogenic A. hydrophila challenge in biocontrol study in vivo as determined by cohabitation challenge. These results suggest that P. aeruginosa FARP72 is a potential probiotic strain and can be used in aquaculture to improve the health status and disease resistance of fish.






Similar content being viewed by others
References
Food and Agriculture Organization of the United Nations (FAO), The state of world fisheries and aquaculture. https://www.fao.org/docrep/016/i2727e/i2727e00.htm., 2012
Austin B, Austin DA (2012) Bacterial fish pathogens: disease of farmed and wild fish, 5th edn. Chichester
Davies J, Davies D (2010) Origins and evolution of antibiotic resistance. Microbiol Mol Biol Rev 74(3):417–433. https://doi.org/10.1128/MMBR.00016-10
Oost RVD, Beyer J, Vermeulen NPE (2003) Fish bioaccumulation and biomarkers in environmental risk assessment: a review. Environ Toxicol Pharmacol 13(2):57–149. https://doi.org/10.1016/S1382-6689(02)00126-6
Nayak SK (2010) Probiotics and immunity: a fish perspective. Fish Shellfish Immunol 29:2–14. https://doi.org/10.1016/j.fsi.2010.02.017
Merrifield DL, Dimitroglou A, Foey A, Davies SJ, Baker RTM, Bogwald J, Castex M, Ringo E (2010) The current status and future focus of probiotic and prebiotic applications for salmonids. Aquaculture 302:1–18. https://doi.org/10.1016/j.aquaculture.2010.02.007
Vieira AT, Teixeira MM, Martins FS (2013) The role of probiotics and prebiotics in inducing gut immunity. Front Immunol 4:445. https://doi.org/10.3389/fimmu.2013.00445
Irianto A, Austin B (2002) Probiotics in aquaculture. J Fish Dis 25:633–642. https://doi.org/10.1046/j.1365-2761.2002.00422.x
Vijayan KK, Bright IS, Jayaprakas NS, Alavandi SV, Somnath Pai S, Preetha R, Rajan JJS, Santiago TC (2005) A brackishwater isolate of Pseudomonas PS-102, a potential antagonistic bacterium against pathogenic vibrios in penaeid and non-penaeid rearing systems. Aquaculture 251:192–200. https://doi.org/10.1016/j.aquaculture.10.010
Chythanya R, Karunasagar I, Karunasagar I (2002) Inhibition of shrimp pathogenic vibrios by a marine Pseudomonas I-2 strain. Aquaculture 208:1–10. https://doi.org/10.1016/S0044-8486(01)00714-1
Giri SS, Sen SS, Sukumaran V (2012) Effects of dietary supplementation of potential probiotic Pseudomonas aeruginosa VSG-2 on the innate immunity and disease resistance of tropical freshwater fish, Labeo rohita. Fish Shellfish Immunol 26:245–248. https://doi.org/10.1016/j.fsi.2012.03.019
Sahu MK, Swarnakumar NS, Sivakumar K, Thangaradjou T, Kannan L (2008) Probiotics in aquaculture: importance and future perspectives. Indian J Microbiol 48:299–308. https://doi.org/10.1007/s12088-008-0024-3
Food and Agriculture Organization of the United Nations/World Health Organization (FAO/WHO) (2002) Guidelines for the evaluation of probiotics in food. Report of a Joint FAO/WHO working group on drafting guidelines for the evaluation of probiotics in food. http://www.fao.org/3/a-a0512e.pdf
Hoque F (2015) Screening and characterisation of antagonistic Pseudomonas aeruginosa FARP72 as a potential probiotic agent. Indian J Fish 62(4):80–90
Austin B, Baudet E, Stobie M (1992) Inhibition of bacterial fish pathogens by Tetraselmis suecica. J Fish Dis 15:55–61. https://doi.org/10.1111/j.1365-2761.1992.tb00636.x
Nakamura A, Takahashi KG, Mori K (1999) Vibriostatic bacteria isolated from rearing seawater of oyster brood stock: potentiality as biocontrol agents for vibriosis in oyster larvae. Fish Pathol 34:139–144. https://doi.org/10.3147/jsfp.34.139
Gram L, Melchiorsen J, Spanggaard B, Huber I, Nielsen TF (1999) Inhibition of Vibrio anguillarum by Pseudomonas fluorescens AH2, a possible probiotic treatment of fish. Appl Environ Microbiol 65(3):969–973
Román L, Real F, Sorroza L, Padilla D, Acosta B, Grasso V, Bravo J, Acosta F (2012) The in vitro effect of probiotic Vagococcus fluvialis on the innate immune parameters of Sparus aurata and Dicentrarchus labrax. Fish Shellfish Immunol 33:1071–1075. https://doi.org/10.1016/j.fsi.2012.06.028
Kamilya D, Ghosh D, Bandyopadhyay S, Mal BC, Maiti TK (2006) In vitro effects of bovine lactoferrin, mushroom glucan and Abrus agglutinin on Indian major carp, catla (Catla catla) head kidney leukocytes. Aquaculture 253:130–139. https://doi.org/10.1016/j.aquaculture.2005.07.038
Strober W (2001) Trypan blue exclusion test of cell viability. Curr Protoc Immunol Appendix 3B. https://doi.org/10.1002/0471142735.ima03bs21
Devi TB, Kamilya D, Abraham TJ (2012) Dynamic changes in immune-effector activities of Indian major carp, catla (Catla catla) infected with Edwardsiella tarda. Aquaculture 366-367:62–66. https://doi.org/10.1016/j.aquaculture.2012.09.002
Kamilya D, Baruah A, Sangma T, Chowdhury S, Pal P (2015) Inactivated probiotic bacteria stimulate cellular immune responses of catla, Catla catla (Hamilton) in vitro. ProbioticsAntimicrobial Proteins 7:101–106. https://doi.org/10.1007/s12602-015-9191-9
Mosmann T (1983) Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods 65:55–63
Madetoja J, Nyman P, Wiklund T (2000) Flavobacterium psychrophilum, invasion into, shedding by rainbow trout Oncorhynchus mykiss. Dis Aquat Org 43:27–38. https://doi.org/10.3354/dao043027
Trivedi P, Pandey A, Palni LS (2008) In vitro evaluation of antagonistic properties of Pseudomonas corrugate. Microbiol Res 163(3):329–336. https://doi.org/10.1016/j.micres.2006.06.007
El-Rhman AMA, Khattab YAE, Shalaby AME (2009) Micrococcus luteus and Pseudomonas species as probiotics for promoting the growth performance and health of Nile tilapia, Oreochromis niloticus. Fish Shellfish Immunol 27:175–180. https://doi.org/10.1016/j.fsi.2009.03.020
Giri SS, Sukumaran V, Sen SS, Vinumonia J, Banu BN, Jena PK (2011) Antagonistic activity of cellular components of potential probiotic bacteria, isolated from the gut of Labeo rohita, against Aeromonas hydrophila. Fish Shellfish Immunol 3:214–222. https://doi.org/10.1007/s12602-011-9078-3
Cazorla FM, Duckett SB, Bergstroem ET, Noreen S, Odijk R, Lugtenberg BJ, Thomas-Oates JE, Bloem-berg GV (2006) Biocontrol of Avocado dematophora root rot by antagonistic Pseudomonas fluorescens PCL1606 correlates with the production of 2-hexyl 5-propyl resorcinol. Mol Plant-Microbe Interact 19:418–428. https://doi.org/10.1094/MPMI-19-0418
Bentley R (1990) From antibiosis to antibiotics a brief account of early work. Biochemist 12(5):14–17
Akya A, Pointon A, Thomas C (2009) Viability of Listeria monocytogenes in co-culture with Acanthamoeba spp. FEMS Microbiol Ecol 70:20–29. https://doi.org/10.1111/j.1574-6941.2009.00736.x
Salinas I, Díaz-Rosales P, Cuesta A, Meseguer J, Chabrillón M, Moriñigo MA, Esteban MA (2006) Effect of heat-inactivated fish and non-fish derived probiotics on the innate immune parameters of a teleost fish (Sparus aurata L.). Vet Immunol Immunopathol 111:279–286. https://doi.org/10.1016/j.vetimm.2006.01.020
Neumann NF, Fagan D, Belosevic M (1995) Macrophage activating factor (s) secreted by mitogen stimulated gold fish kidney leukocytes synergize with bacterial lypopolysaccharide to induce nitric oxide production in teleost macrophages. Dev Comp Immunol 19:473–482. https://doi.org/10.1016/0145-305X(95)00032-O
Sakai M (1997) Current research status of fish immunostimulants. Aquaculture 172:63–92. https://doi.org/10.1016/S0044-8486(98)00436-0
Abraham TJ, Babu CHS, Mondal S, Banerjee T (2007) Effects of dietary supplementation of commercial human probiotic and antibiotic on the growth rate and content of intestinal microflora in ornamental fishes. Bangladesh J Fish Res 11:57–63
Brunt J, Austin B (2005) Use of a probiotic to control lactococcosis and streptococcosis in rainbow trout, Oncorhynchus mykiss (Walbaum). J Fish Dis 28:693–701. https://doi.org/10.1111/j.1365-2761.2005.00672.x
Taoka Y, Maeda H, Jo JY, Kim SM, Park SI, Yoshikawa T, Sakata T (2006) Use of live and dead probiotic cells in tilapia Oreochromis niloticus. Fish Sci 72:755–766. https://doi.org/10.1111/j.1444-2906.2006.01215.x
Das A, Nakhro K, Chowdhury S, Kamilya D (2013) Effects of potential probiotic Bacillus amyloliquifaciens FPTB16 on systemic and cutaneous mucosal immune responses and disease resistance of catla (Catla catla). Fish Shellfish Immunol 35:1547–1553. https://doi.org/10.1016/j.fsi.2013.08.022
Acknowledgements
The authors thank the Vice-Chancellor, West Bengal University of Animal and Fishery Sciences, Kolkata for providing necessary infrastructure facility to carry out the work.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical Statement
All authors of this paper have read and approved the final version submitted. The contents of this manuscript have not been copyrighted or published previously.
1. The contents of this manuscript are not now under consideration for publication elsewhere.
2. The contents of this manuscript will not be copyrighted, submitted, or published elsewhere, while acceptance by the Journal is under consideration.
3. Use of laboratory animals (fish) in the present study has complied with the guidelines and policies of the ethical committee of the Institute.
Conflict of Interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hoque, F., Jawahar Abraham, T., Nagesh, T.S. et al. Pseudomonas aeruginosa FARP72 Offers Protection Against Aeromonas hydrophila Infection in Labeo rohita. Probiotics & Antimicro. Prot. 11, 973–980 (2019). https://doi.org/10.1007/s12602-018-9456-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12602-018-9456-1