Abstract
Platinum (Pt) is a critical raw material for automotive catalytic converters due to its high-temperature stability, corrosion resistance and catalytic activity, whereas its limited primary resources and uneven distribution make it hard to meet the growing demand of platinum. Spent automotive catalyst (SAC) is currently the most important secondary resource of platinum, of which the platinum content is much higher than that of the primary platinum resources. The recovery process of platinum from spent automobile catalyst mainly consists of pretreatment followed by enrichment and refining, involving pyro- and hydrometallurgical techniques, among which enrichment and refining processes are extremely important for platinum recovery from spent automobile catalyst. This paper provides an overview of the technologies for platinum recovery from spent automotive catalyst. The emphasis is placed on the processes of enrichment and refining based on hydrometallurgical techniques. Future directions of research and development of platinum recovery from spent automobile catalyst are also proposed.
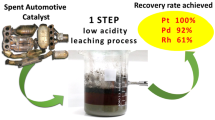
Graphical Abstract

摘要
金属铂具有优异的物化性能,是目前汽车催化转化器的关键原材料。含铂矿产资源储量少、分布不均且成分复杂,难以满足其日益增长的需求。废汽车催化剂作为主要的含铂二次资源,产生量大、铂含量高,其高效回收可有效缓解铂的供需压力。从废汽车催化剂中回收铂,主要包括预处理、富集和精炼三大工序,涉及火法和湿法冶金处理工艺。本文基于湿法冶金,对从废汽车催化剂中回收铂的富集和精炼工艺技术进行综述,并进一步提出从废汽车尾气催化剂中回收铂的发展建议。





Similar content being viewed by others
References
Cowley A. Platinum 2013 interim review. Johns Matthey. 2013: 1. https://doi.org/10.1016/S1351-4180(13)70483-5
Jha MK, Lee JC, Kim MS, Jeong J, Kim BS, Kumar V. Hydrometallurgical recovery/recycling of platinum by the leaching of spent catalysts: a review. Hydrometall. 2013;133:23. https://doi.org/10.1016/j.hydromet.2012.11.012.
Qin YC, Wang FQ, Wang XM, Wang MW, Zhang WL, An WK, Wang XP, Ren YL, Zheng X, Lv DC, Ahmad A. Noble metal-based high-entropy alloys as advanced electrocatalysts for energy conversion. Rare Met. 2021;40(9):2354. https://doi.org/10.1007/s12598-021-01727-y.
Omrani M, Goriaux M, Liu Y, Martinet S, Jean-Soro L, Ruban V. Platinum group elements study in automobile catalysts and exhaust gas samples. Environ Pollut. 2020;257:113477. https://doi.org/10.1016/j.envpol.2019.113477.
Chen Y, Qiao QY, Cao JZ, Li HX, Bian ZF. Precious metal recovery. Joule. 2021;5(12):3097. https://doi.org/10.1016/j.joule.2021.11.002.
Cowley A. PGM market report: May 2022. Johnson Matthey. 2022.
Reith F, Campbell SG, Ball AS, Pring A, Southam G. Platinum in earth surface environments. Earth-Sci Rev. 2014;131:1. https://doi.org/10.1016/j.earscirev.2014.01.003.
Cabri LJ, Oberthür T, Keays RR. Origin and depositional history of platinum-group minerals in placers-a critical review of facts and fiction. Ore Geol Rev. 2022;144: 104733. https://doi.org/10.1016/j.oregeorev.2022.104733.
Karim S, Ting YP. Recycling pathways for platinum group metals from spent automotive catalyst: a review on conventional approaches and bio-processes. Resour Conserv Recycl. 2021;170:105588. https://doi.org/10.1016/j.resconrec.2021.105588.
Bahaloo-Horeh N, Mousavi SM. Comprehensive characterization and environmental risk assessment of end-of-life automotive catalytic converters to arrange a sustainable roadmap for future recycling practices. J Hazard Mater. 2020;400: 123186. https://doi.org/10.1016/j.jhazmat.2020.123186.
Lucena P, Vadillo JM, Laserna JJ. Mapping of platinum group metals in automotive exhaust three-way catalysts using laser-Induced breakdown spectrometry. Anal Chem. 1999;71(19):4385. https://doi.org/10.1021/ac9902998.
Li GF, Wang QY, Zhao B, Shen MQ, Zhou RX. Effect of iron doping into CeO2-ZrO2 on the properties and catalytic behaviour of Pd-only three-way catalyst for automotive emission control. J Hazard Mater. 2011;186(1):911. https://doi.org/10.1016/j.jhazmat.2010.11.080.
Shan WP, Liu FD, Yu YB, He H. The use of ceria for the selective catalytic reduction of NOx with NH3. Chin J Catal. 2014;35(8):1251. https://doi.org/10.1016/S1872-2067(14)60155-8.
Cui MS, Zhang YQ, Zhong Q, Long ZQ, Zhao N, Huang XW. Portable XRF analysis of noble metal contents for automotive catalysts. Chin J Rare Met. 2020;44(11):1227. https://doi.org/10.13373/j.cnki.cjrm.XY180900111.
Nevalainen P, Kinnunen NM, Kirveslahti A, Kallinen K, Maunula T, Keenan M, Suvanto M. Formation of NH3 and N2O in a modern natural gas three-way catalyst designed for heavy-duty vehicles: the effects of simulated exhaust gas composition and ageing. Appl Catal A. 2018;552:30. https://doi.org/10.1016/j.apcata.2017.12.017.
Hickey N, Boscarato I, Kaspar J. Current Environmental Issues and Challenges. In: Cao G, Orrù R, editors. Dordrecht: Springer; 2014. 15. https://doi.org/10.1007/978-94-017-8777-2.
Morikawa A, Okumura K, Ishii M, Kikuta K, Suda A, Shinjo H. Characterization of termetallic Pt-Ir-Au catalysts for NO decomposition. Rare Met. 2011;30(1):53. https://doi.org/10.1007/s12598-011-0196-6.
Faisal M, Atsuta Y, Daimon H, Fujie K. Recovery of precious metals from spent automobile catalytic converters using supercritical carbon dioxide. Asia-Pac J Chem Eng. 2008;3(4):364. https://doi.org/10.1002/apj.156.
Zheng HD, Ding YJ, Wen Q, Liu B, Zhang SG. Separation and purification of platinum group metals from aqueous solution: Recent developments and industrial applications. Resour Conserv Recycl. 2021;167:105417. https://doi.org/10.1016/j.resconrec.2021.105417.
Rzelewska-Piekut M, Paukszta D, Regel-Rosocka R. Hydrometallurgical recovery of platinum group metals from spent automotive converters. Physicochem Probl Min Process. 2021;57(2):83. https://doi.org/10.37190/ppmp/132779.
Dong HG, Zhao JC, Chen JL, Wu YD, Li BJ. Recovery of platinum group metals from spent catalysts: a review. Int J Min Process. 2015;145:108. https://doi.org/10.1016/j.minpro.2015.06.009.
Liu C, Sun SC, Zhu XP, Tu GF. Metals smelting-collection method for recycling of platinum group metals from waste catalysts: a mini review. Waste Manage Res. 2021;39(1):43. https://doi.org/10.1177/0734242X20969795.
Granados-Fernández R, Montiel MA, Díaz-Abad S, Rodrigo MA, Lobato J. Platinum recovery techniques for a circular economy. Catal. 2021;11(8):937. https://doi.org/10.3390/catal11080937.
Kim CH, Woo SI, Jeon SH. Recovery of platinum-group metals from recycled automotive catalytic converters by carbochlorination. Ind Eng Chem Res. 2000;39(5):1185. https://doi.org/10.1021/ie9905355.
Yakoumis I, Panou M, Moschovi AM, Panias D. Recovery of platinum group metals from spent automotive catalysts: a review. Clea Eng Technol. 2021;3: 100112. https://doi.org/10.1016/j.clet.2021.100112.
Kolliopoulos G, Balomenos E, Giannopoulou I, Yakoumis I, Panias D. Behavior of platinum group during their pyrometallurgical recovery from spent automotive catalysts. Open Access Librar J. 2014;1(5):1. https://doi.org/10.4236/oalib.1100736.
Ding YJ, Zheng HD, Zhang SG, Liu B, Wu BY, Jian ZM. Highly efficient recovery of platinum, palladium, and rhodium from spent automotive catalysts via iron melting collection. Resour Conserv Recycl. 2020;155:104644. https://doi.org/10.1016/j.resconrec.2019.104644.
Compernolle S, Wambeke D, De Raedt I, Kimpe K, Vanhaecke F. Direct determination of Pd, Pt and Rh in fire assay lead buttons by laser ablation-ICP-OES: automotive exhaust catalysts as an example. J Anal At Spectrom. 2011;26(8):1679. https://doi.org/10.1039/C1JA10079C.
Morcali MH. A new approach to recover platinum-group metals from spent catalytic converters via iron matte. Resour Conserv Recycl. 2020;159:104891. https://doi.org/10.1016/j.resconrec.2020.104891.
Zhang FY, Zhang GA, Xu L, Zhao Z. Enrichment of Pd, Pt and Rh from spent automotive catalyst by pyrometallurgical bismuth capture. Chin J Nonferrous Metals. 2020;30(9):2162. https://doi.org/10.11817/j.ysxb.1004.0609.2020-36475.
Peng ZW, Li ZZ, Lin XL, Tang HM, Ye L, Ma YT, Rao MJ, Zhang YB, Li GH, Jiang T. Pyrometallurgical recovery of platinum group metals from spent catalysts. JOM. 2017;69(9):1553. https://doi.org/10.1007/s11837-017-2450-3.
Trinh HB, Lee JC, Suh YJ, Lee J. A review on the recycling processes of spent auto-catalysts: towards the development of sustainable metallurgy. Waste Manage. 2020;114:148. https://doi.org/10.1016/j.wasman.2020.06.030.
Ding L, Yang JG, Yan WP, Li SC, Nan TX, Li LC. Enrichment of platinum group metals from cordierite-type automotive exhaust catalyst. Hydrometall Chin. 2018;37(5):376. https://doi.org/10.13355/j.cnki.sfyj.2018.05.008.
Trinh HB, Lee J, Srivastava RR, Kim S. Total recycling of all the components from spent auto-catalyst by NaOH roasting-assisted hydrometallurgical route. J Hazard Mater. 2019;379: 120772. https://doi.org/10.1016/j.jhazmat.2019.120772.
Kim MS, Park SW, Lee JC, Choubey PK. A novel zero emission concept for electrogenerated chlorine leaching and its application to extraction of platinum group metals from spent automotive catalyst. Hydrometall. 2016;159:19. https://doi.org/10.1016/j.hydromet.2015.10.030.
Kim MS, Lee JC, Park SW, Jeong J, Kumar V. Dissolution behaviour of platinum by electro-generated chlorine in hydrochloric acid solution. J Chem Technol Biotechnol. 2013;88(7):1212. https://doi.org/10.1002/jctb.3957.
Upadhyay AK, Lee JC, Kim EY, Kim MS, Kim BS, Kumar V. Leaching of platinum group metals (PGMs) from spent automotive catalyst using electro-generated chlorine in HCl solution. J Chem Technol Biotechnol. 2013;88(11):1991. https://doi.org/10.1002/jctb.4057.
Kizilaslan E, Aktaş S, Şeşen MK. Towards environmentally safe recovery of platinum from scrap automotive catalytic converters. Turk J Eng Env Sci. 2009;33(2):83. https://doi.org/10.3906/muh-0901-10.
De Aberasturi DJ, Pinedo R, De Larramendi IR, De Larramendi JIR, Rojo T. Recovery by hydrometallurgical extraction of the platinum-group metals from car catalytic converters. Min Eng. 2011;24(6):505. https://doi.org/10.1016/j.mineng.2010.12.009.
Yakoumis I, Moschovi A, Panou M, Panias D. Single-step hydrometallurgical method for the platinum group metals leaching from commercial spent automotive catalysts. J Sustain Metall. 2020;6(2):259. https://doi.org/10.1007/s40831-020-00272-9.
Ding YJ, Zhang SG, Liu B, Zheng HD, Chang CC, Ekberg C. Recovery of precious metals from electronic waste and spent catalysts: a review. Resour Conserv Recy. 2019;141:284. https://doi.org/10.1016/j.resconrec.2018.10.041.
Wang JX, Faraji F, Ramsay J, Ghahreman A. A review of biocyanidation as a sustainable route for gold recovery from primary and secondary low-grade resources. J Clean Prod. 2021;296:126457. https://doi.org/10.1016/j.jclepro.2021.126457.
Sun SQ, Jin CX, He WZ, Li GM, Zhu HC, Huang JW. A review on management of waste three-way catalysts and strategies for recovery of platinum group metals from them. J Environ Manage. 2022;305:114383. https://doi.org/10.1016/j.jenvman.2021.114383.
Atkinson GB, Kuczynski RJ, Desmond DP. Cyanide leaching method for recovering platinum group metals from a catalytic converter catalyst. US. Patent; US5160711A. 1992.
Shams K, Beiggy MR, Shirazi AG. Platinum recovery from a spent industrial dehydrogenation catalyst using cyanide leaching followed by ion exchange. Appl Catal A. 2004;258(2):227. https://doi.org/10.1016/j.apcata.2003.09.003.
Chen J, Huang K. A new technique for extraction of platinum group metals by pressure cyanidation. Hydrometall. 2006;82(3–4):164. https://doi.org/10.1016/j.hydromet.2006.03.041.
Naghavi Z, Ghoreishi SM, Rahimi A, Hadadzadeh H. Kinetic study for platinum extraction from spent catalyst in cyanide solution at high temperatures. Int J Chem React Eng. 2016;14(1):143. https://doi.org/10.1515/ijcre-2015-0046.
Karim S, Ting YP. Ultrasound-assisted nitric acid pretreatment for enhanced biorecovery of platinum group metals from spent automotive catalyst. J Clean Prod. 2020;255:120199. https://doi.org/10.1016/j.jclepro.2020.120199.
Ilyas S, Kim H. Recovery of platinum-group metals from an unconventional source of catalytic converter using pressure cyanide leaching and ionic liquid extraction. JOM. 2022;74(3):1020. https://doi.org/10.1007/s11837-021-05119-6.
Saguru C, Ndlovu S, Moropeng D. A review of recent studies into hydrometallurgical methods for recovering PGMs from used catalytic converters. Hydrometall. 2018;182:44. https://doi.org/10.1016/j.hydromet.2018.10.012.
Donato DB, Nichols O, Possingham H, Moore M, Ricci PF, Noller BN. A critical review of the effects of gold cyanide-bearing tailings solutions on wildlife. Environ Int. 2007;33(7):974. https://doi.org/10.1016/j.envint.2007.04.007.
Huang SW, Fei WL, Zhang MJ, Yao QC. A method for extraction and refining of platinum group metals from spent automobile catalyst. China Patent; CN201710780442.6. 2018.
Hammadi MQ, Yassen RS, Abid KN. Recovery of platinum and palladium from scrap automotive catalytic converters. Al-Khwarizmi Eng J. 2017;13(3):131. https://doi.org/10.22153/kej.2017.04.002.
Zhao JC, Wu YD, Tong WF, Yang HQ, Bao SM, Pei HY, Dong HG. Research progress on preparation of high purity platinum. Precious Met. 2020;41(1):92. https://doi.org/10.3969/j.issn.1004-0676.2020.01.015.
Liu SJ. Metallurgy of platinum group metals. Changsha, Hunan: Central South University Press; 2013. 498.
Lee JC, Kurniawan Hong HJ, Chung KW, Kim S. Separation of platinum, palladium and rhodium from aqueous solutions using ion exchange resin: a review. Sep Purif Technol. 2020;246:116896. https://doi.org/10.1016/j.seppur.2020.116896.
Barbaro P, Liguori F. Ion exchange resins: catalyst recovery and recycle. Chem Rev. 2009;109(2):515. https://doi.org/10.1021/cr800404j.
Nikoloski AN, Ang KL. Review of the application of ion exchange resins for the recovery of platinum-group metals from hydrochloric acid solutions. Min Process Extr Metall Rev. 2014;35(6):369. https://doi.org/10.1080/08827508.2013.764875.
Luo Y, He X, Xiong XD, Chen F, Wu C, Chen Q, Li YT. Mechanism of removing impurities in high purity platinum using ion exchange. Precious Met. 2013;34(S1):1.
Bi XG, Yu JM, Yang JF, He HL, Li Q. Purification of platinum group metals solution by cation exchange. Min Metall Eng. 2015;35(3):87. https://doi.org/10.3969/j.issn.0253-6099.2015.03.024.
Zhao DP, Yang RS, Zou AQ, Wang SX, Guo H, Yang XJ. Adsorption of low concentration platinum from chloride leaching solution with 201×7 ion-exchange resin. Precious Met. 2014;35(2):43. https://doi.org/10.3969/j.issn.1004-0676.2014.02.010.
Shen SB, Guishen L, Pan TL, He JZ, Guo ZC. Selective adsorption of Pt ions from chloride solutions obtained by leaching chlorinated spent automotive catalysts on ion exchange resin Diaion WA21J. J Colloid Interface Sci. 2011;364(2):482. https://doi.org/10.1016/j.jcis.2011.08.043.
Kononova ON, Melnikov AM, Borisova TV, Krylov AS. Simultaneous ion exchange recovery of platinum and rhodium from chloride solutions. Hydrometall. 2011;105(3–4):341. https://doi.org/10.1016/j.hydromet.2010.11.009.
Schoeman E, Bradshaw SM, Akdogan G, Snyders CA, Eksteen JJ. The extraction of platinum and palladium from a synthetic cyanide heap leach solution with strong base anion exchange resins. Int J Min Process. 2017;162:27. https://doi.org/10.1016/j.minpro.2017.02.017.
Huang HL, Qu RJ, Sun CM, Zhang Y. Adsorption properties for Pt(IV) of a novel chelating resin polystyrene-supported glucosamine. Ion Exch Adsorpt. 2010;26(5):385.
Nikoloski AN, Ang KL, Li D. Recovery of platinum, palladium and rhodium from acidic chloride leach solution using ion exchange resins. Hydrometall. 2015;152:20. https://doi.org/10.1016/j.hydromet.2014.12.006.
Dobrowolski R, Mróz A, Cejner M. The enrichment of Pt(IV) ions on Dowex-1X8 and Purolite S-920 ion exchangers from aqueous solutions and their determination using slurry sampling and direct solid sampling graphite furnace atomic absorption spectrometry techniques. Anal Methods. 2016;8(29):5818. https://doi.org/10.1039/c6ay00998k.
Morcali MH, Zeytuncu B. Investigation of adsorption parameters for platinum and palladium onto a modified polyacrylonitrile-based sorbent. Int J Min Process. 2015;137:52. https://doi.org/10.1016/j.minpro.2015.02.011.
Zhou LM, Xu JP, Liang XZ, Liu ZR. Adsorption of platinum(IV) and palladium(II) from aqueous solution by magnetic cross-linking chitosan nanoparticles modified with ethylenediamine. J Hazard Mater. 2010;182(1–3):518. https://doi.org/10.1016/j.jhazmat.2010.06.062.
Xu ZX, Zhao YL, Wang PY, Yan XQ, Cai MM, Yang Y. Extraction of Pt(IV), Pt(II), and Pd(II) from acidic chloride media using imidazolium-based task-specific polymeric ionic liquid. Ind Eng Chem Res. 2019;58(5):1779. https://doi.org/10.1021/acs.iecr.8b03408.
Xiong CH, Zheng YQ, Feng YJ, Yao CP, Ma CA, Zheng XM, Jiang JX. Preparation of a novel chloromethylated polystyrene-2-amino-1,3,4-thiadiazole chelating resin and its adsorption properties and mechanism for separation and recovery of Pt(IV) from aqueous solutions. J Mater Chem A. 2014;2(15):5379. https://doi.org/10.1039/c3ta14923d.
Izatt SR, Bruening RL, Izatt NE. Metal separations and recovery in the mining industry. JOM. 2012;64(11):1279. https://doi.org/10.1007/s11837-012-0452-8.
Izatt RM, Izatt SR, Izatt NE, Krakowiak KE, Bruening RL, Navarro L. Industrial applications of molecular recognition technology to separations of platinum group metals and selective removal of metal impurities from process streams. Green Chem. 2015;17(4):2236. https://doi.org/10.1039/c4gc02188f.
Papaiconomou N, Svecova L, Bonnaud C, Cathelin L, Billard I, Chainet E. Possibilities and limitations in separating Pt(IV) from Pd(II) combining imidazolium and phosphonium ionic liquids. Dalton Trans. 2015;44(46):20131. https://doi.org/10.1039/C5DT03791C.
Jaree A, Khunphakdee N. Separation of concentrated platinum(IV) and rhodium(III) in acidic chloride solution via liquid-liquid extraction using tri-octylamine. J Ind Eng Chem. 2011;17(2):243. https://doi.org/10.1016/j.jiec.2011.02.013.
Swain B, Jeong J, Kim SK, Lee JC. Separation of platinum and palladium from chloride solution by solvent extraction using Alamine 300. Hydrometall. 2010;104(1):1. https://doi.org/10.1016/j.hydromet.2010.03.013.
Lee JY, Kumar JR, Kim JS, Park HK, Yoon HS. Liquid-liquid extraction/separation of platinum(IV) and rhodium(III) from acidic chloride solutions using tri-iso-octylamine. J Hazard Mater. 2009;168(1):424. https://doi.org/10.1016/j.jhazmat.2009.02.056.
Baba Y, Arima A, Kanemaru S, Iwakuma M, Oshima T. Extraction equilibria of palladium(II) and platinum(IV) with N, N-di(2-ethylhexyl)aminomethylquinoline from hydrochloric acid. J Chem Eng Jpn. 2011;44(10):686. https://doi.org/10.1252/jcej.10we284.
Maeda M, Narita H, Tokoro C, Tanaka M, Motokawa R, Shiwaku H, Yaita T. Selective extraction of Pt(IV) over Fe(III) from HCl with an amide-containing tertiary amine compound. Sep Purif Technol. 2017;177:176. https://doi.org/10.1016/j.seppur.2017.01.002.
Liu WH, Wang Q, Zheng Y, Wang SB, Yan Y, Yang YZ. Extraction behaviour and mechanism of Pt(IV) and Pd(II) by liquid-liquid extraction with an ionic liquid [HBBIm]Br. Dalton Trans. 2017;46(22):7210. https://doi.org/10.1039/c7dt01142c.
Wang N, Wang Q, Lu WJ, Ru MY, Yang YZ. Extraction and stripping of platinum (IV) from acidic chloride media using guanidinium ionic liquid. J Mol Liq. 2019;293: 111040. https://doi.org/10.1016/j.molliq.2019.111040.
Zhao Z, Xiong YH, Cheng XK, Hou X, Yang YX, Tian YP, You JL, Xu L. Adsorptive removal of trace thallium(I) from wastewater: a review and new perspectives. J Hazard Mater. 2020;393: 122378. https://doi.org/10.1016/j.jhazmat.2020.122378.
Yamada M, Rajiv Gandhi M, Kondo Y, Hamada F. Synthesis and characterisation of p-diethylaminomethylthiacalix[4]arene for selective recovery of platinum from automotive catalyst residue. Supramol Chem. 2014;26(7–8):620. https://doi.org/10.1080/10610278.2014.887202.
Yamada M, Rajiv Gandhi M, Kaneta Y, Hu Y, Shibayama A. Calix[4]arene-based n-dialkylamino extractants for selective platinum group metal separation from automotive catalysts. Chem Select. 2017;2(3):1052. https://doi.org/10.1002/slct.201601981.
Yamada M, Kaneta Y, Rajiv Gandhi M, Kunda UMR, Shibayama A. Calix[4]arene-based amino extractants containing n-alkyl moieties for separation of Pd(II) and Pt(IV) from leach liquors of automotive catalysts. Metals. 2018;8(7):517. https://doi.org/10.3390/met8070517.
Yamada M, Kaneta Y, Rajiv Gandhi M, Kunda UMR, Shibayama A. Recovery of Pd(II) and Pt(IV) from leach liquors of automotive catalysts with calixarene-based di-n-alkylamino extractants in saturated hydrocarbon diluents. Hydrometall. 2019;184:103. https://doi.org/10.1016/j.hydromet.2019.01.002.
Sulaiman RNR, Othman N, Jusoh N, Noah NFM, Rashid R, Saufi SM. Intensification reactive recovery of tetravalent platinum from spent catalyst via synergism of TBP/Cyanex 302 system. Chem Eng Process. 2021;168: 108581. https://doi.org/10.1016/j.cep.2021.108581.
Gupta B, Singh I. Extraction and separation of platinum, palladium and rhodium using Cyanex 923 and their recovery from real samples. Hydrometall. 2013;134–135:11. https://doi.org/10.1016/j.hydromet.2013.01.001.
Ilyas S, Kim H, Srivastava RR. Separation of platinum group metals from model chloride solution using phosphonium-based ionic liquid. Sep Purif Technol. 2022;278: 119577. https://doi.org/10.1016/j.seppur.2021.119577.
Rzelewska-Piekut M, Regel-Rosocka M. Separation of Pt(IV), Pd(II), Ru(III) and Rh(III) from model chloride solutions by liquid-liquid extraction with phosphonium ionic liquids. Sep Purif Technol. 2019;212:791. https://doi.org/10.1016/j.seppur.2018.11.091.
Liu RH, Geng YQ, Tian ZJ, Wang N, Wang M, Zhang GJ, Yang YZ. Extraction of platinum(IV) by hydrophobic deep eutectic solvents based on trioctylphosphine oxide. Hydrometall. 2021;199:105521. https://doi.org/10.1016/j.hydromet.2020.105521.
Chen ZH. Study on behaviour and mechanism of extraction of platinum(IV) with unsymmetrical sulfoxide BSO. Rare Metal Meter Eng. 2009;38(6):1062. https://doi.org/10.3321/j.issn:1002-185X.2009.06.027.
Chen ZH, Gu GB, Zuo H, Wu F. Study on extraction and separation of palladium and platinum with unsymmetrical sulfoxide BSO. Nonferrous Metals (Extr Metall). 2012;7:34. https://doi.org/10.3969/j.issn.1007-7545.2012.07.009.
Pan L, Zhang ZD. Solvent extraction and separation of palladium(II) and platinum(IV) from hydrochloric acid medium with dibutyl sulfoxide. Min Eng. 2009;22(15):1271. https://doi.org/10.1016/j.mineng.2009.07.006.
Gupta B, Singh I, Mahandra H. Extraction and separation studies on Pt(IV), Ir(III) and Rh(III) using sulphur containing extractant. Sep Purif Technol. 2014;132:102. https://doi.org/10.1016/j.seppur.2014.04.045.
Zhang GJ, Zhang LX, Wang Q, Guo JX, Wei HY, Yang YZ. Extraction and separation of Pd(II)/Pt(IV) by neutral sulfur-containing extractant from hydrochloric acid medium. New J Chem. 2021;45(41):19467. https://doi.org/10.1039/d1nj03140f.
He KB, Tang J, Weng HQ, Chen G, Wu ZH, Lin MZ. Efficient extraction of precious metal ions by a membrane emulsification circulation extractor. Sep Purif Technol. 2019;213:93. https://doi.org/10.1016/j.seppur.2018.12.024.
Jha R, Rao MD, Meshram A, Verma HR, Singh KK. Potential of polymer inclusion membrane process for selective recovery of metal values from waste printed circuit boards: a review. J Clean Prod. 2020;265:121621. https://doi.org/10.1016/j.jclepro.2020.121621.
Liu L, Liu SX, Zhang QP, Li C, Bao CL, Liu XT, Xiao PF. Adsorption of Au(III), Pd(II), and Pt(IV) from aqueous solution onto graphene oxide. J Chem Eng Data. 2013;58(2):209. https://doi.org/10.1021/je300551c.
Li K, Xu ZM. A review of current progress of supercritical fluid technologies for e-waste treatment. J Clean Prod. 2019;227:794. https://doi.org/10.1016/j.jclepro.2019.04.104.
Wang SY, Vincent T, Roux J, Faur C, Guibal E. Pd(II) and Pt(IV) sorption using alginate and algal-based beads. Chem Eng J. 2017;313:567. https://doi.org/10.1016/j.cej.2016.12.039.
Garole DJ, Choudhary BC, Paul D, Borse AU. Sorption and recovery of platinum from simulated spent catalyst solution and refinery wastewater using chemically modified biomass as a novel sorbent. Environ Sci Pollut Res. 2018;25(11):10911. https://doi.org/10.1007/s11356-018-1351-5.
Acknowledgements
This study was financially supported by the Natural Science Foundation of Anhui Province (No. 2108085J26), the National Natural Science Foundation of China (Nos. 51904003 and U1703130), the Key Research and Development Plan of Anhui Province (No. 2022n07020004) and the Open Foundation of State Key Laboratory of Complex Nonferrous Metal Resources Clean Utilization (No. CNMRCUKF2208).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no conflict of interest.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Ge, T., He, JD., Xu, L. et al. Recovery of platinum from spent automotive catalyst based on hydrometallurgy. Rare Met. 42, 1118–1137 (2023). https://doi.org/10.1007/s12598-022-02236-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-022-02236-2