Abstract
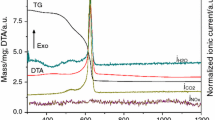
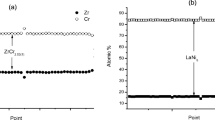
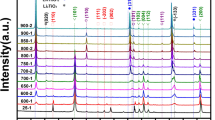
A study was carried out on the preparation of YAl2 intermetallics from mixed oxide precursors using the method of electro-deoxidation. Y2O3 and Al2O3 mixed in molar proportions of 1:2 were sintered at temperatures ranging from 800 to 1200 °C for different hours. The sintered pellet was then electrolyzed in a molten CaCl2 at 850 °C using a graphite anode at a potential of 3.1 V. In this work, effects of sintering on the composition and the oxygen content in cathodic products were studied by X-ray diffraction (XRD) and oxygen–nitrogen analyzer. Sintering mainly affects the porosity of the pellets, and it is found that porosity decreases gradually with the increase in sintering temperature and the extension of sintering time. When sintered at 900 °C for 4 h, the oxygen content in the cathodic product decreases to 1.85 wt%, and the efficiency of electro-deoxidation is 94.48%. At the same time, the mechanism of electrolysis was speculated. The results suggest that the formation of YAl2 is a multi-step process. During the reduction process from mixed oxides Y2O3/Al2O3 to YAl2 included many intermediate materials, such as YAlO3, Y3Al5O12 and YAl3, YAl2 intermediate alloy was prepared finally.














Similar content being viewed by others
References
Lei GX. Effect and application of rare earth in aluminum and aluminum alloys. Light Alloy Process Technol. 1990;18(2):5.
Li GR, Wang HM, Zhao YT. Effect of rare earth yttrium on the melting and solidification process of 7055 aluminum alloy. Rare Met Mater Eng. 2010;39(1):80.
Zhou XX, Zhang RY. Effect and application of rare earth elements in aluminum alloys. New Technol New Process. 2003;4:43.
Li YG, Duan JH, Liu HL. Strengthening effect of yttrium rare earth in Mg–Zn–Zr magnesium alloy. Modern Mach. 2003;5:86.
Pang SM, Yan SH, Li ZA, Chen DH, Xu LH, Zhao B. Development on molten salt electrolytic methods and technology for preparing rare earth metals and alloys in China. Chin J Rare Met. 2011;35(3):440.
He S, Li ZA, Yan SH, Wang ZQ, Pang SM, Chen BY. Cathode process in electrolytic codeposition of Y–Mg alloy in molten fluoride. J Chin Rare Earth Soc. 2007;25(1):120.
Das SK, Davis LA. High performance aerospace alloys via rapid solidification processing. Mater Sci Eng. 1988;98:1.
Harata M. Electrochemical production of Al–Sc alloy in CaCl2–Sc2O3 molten salt. J Alloys Compd. 2009;474(1–2):124.
Tang H, Yan YD, Zhang ML, Li X, Huang Y, Xu YL. AlCl3-aided extraction of praseodymium from Pr6O11 in LiCl–KCl eutectic melts. Electrochim Acta. 2013;88:457.
Suryanarayana C. Mechanical alloying and milling. Prog Mater Sci. 2001;46(1):1.
Chen GZ, Fray DJ, Farthing TW. Direct electrochemical reduction of titanium dioxide to titanium in molten calcium chloride. Nature. 2000;407(6802):361.
Chen GZ, Fray DJ. Voltammetric studies of the oxygen-titanium binary system in molten calcium chloride. J Electrochem Soc. 2002;149(11):E455.
Chen GZ, Fray DJ. Reduction of titanium and other metal oxides using electrodeoxidation. Mater Sci Technol. 2004;20(3):295.
Okabe TH, Mitsuda Y. Titanium powder production by perform reduction process (PRP). J Alloys Compd. 2004;634(1):156.
Okabe TH, Abiko T. Production of titanium powder directly from TiO2 in CaCl2 through an electronically mediated reaction (EMR). J Phys Chem Solids. 2005;66(2):410.
Schwandt C, Doughty GR, Fray DJ. The FFC-Cambridge process for titanium metal winning. Key Eng Mater. 2010;436:13.
Alexander DTL, Schwandt C, Fray DJ. Microstructural kinetics of phase transformations during electrochemical reduction of titanium dioxide in molten calcium chloride. Acta Mater. 2006;54(11):2933.
Okabe TH, Deura TN, Oishi T. Electrochemical deoxidation of yttrium–oxygen solid solutions. J Alloys Compd. 1996;237(1):150.
Anik M, Aybar AB. Synthesis of La2Ni7 hydrogen storage alloy by the electro-deoxidation technique. Int J Hydrogen Energy. 2015;40(5):2248.
Zhu Y, Wang DH, Ma M. More affordable electrolytic LaNi5-type hydrogen storage powders. Chem Commun. 2007;24:2515.
Qiu GH, Wang DH, Ma M, Jin XB, Chen GZ. Electrolytic synthesis of TbFe2 from Tb4O7 and Fe2O3 powders in molten CaCl2. J Electroanal Chem. 2006;589(1):139.
Yin HY, Yu T, Tang DY, Ruan XF, Zhu H, Wang DH. Electrochemical preparation of NiAl intermetallic compound from solid oxides in molten CaCl2 and its corrosion behaviors in NaCl aqueous solution. Mater Chem Phys. 2012;133(1):465.
Yan XY, Fray DJ. Synthesis of niobium aluminides by electro-deoxidation of oxides. J Alloys Compd. 2009;486(1–2):154.
Trulove PC. Molten salts XIII. Pennington: The Electrochemical Society, Inc.; 2002. 745.
Gao BL, Zhu H, Xia YQ. Direct preparation of Al-base alloys from their oxides/metal precursors in the eutectic LiCl–KCl melt. J Alloys Compd. 2016;665:124.
Tan S, Örs T. Synthesis of FeTi from mixed oxide precursors. J Alloys Compd. 2009;475(1–2):368.
Deng LQ. Preparation of niobium and Nb–Ti alloy by electro-deoxidation in a eutectic melt. Shenyang: Northwest University; 2006. 26.
Wang BX, Zhou L, Lan XZ, Zhao XC, Cui JT. Cathode preparation of electrochemical reduction process of TiO2 to titanium. Rare Met Mater Eng. 2010;39(9):1513.
Zhao BJ, Lu XG, Zhong QD, Li CH, Chen SL. Direct electrochemical preparation of CeNi5 and LaxCe1−xNi5 alloys from mixed oxides by SOM process. Electrochim Acta. 2010;55(8):2996.
Acknowledgements
This study was financially supported by the Major State Basic Research Development Program of China (No. 2012BA01207).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wei, YS., Yan, SH., Zhou, L. et al. Formation mechanism and preparation of YAl2 intermetallics by electro-deoxidation with different sintering conditions. Rare Met. 42, 1067–1074 (2023). https://doi.org/10.1007/s12598-016-0845-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0845-x