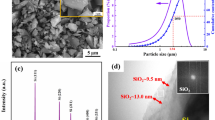
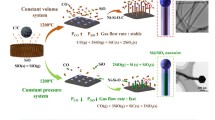
Abstract
Silicon nanowires (SiNWs) with diameter distributions ranging from 80 to 350 nm were prepared by electrochemical reduction of Ni/SiO2 in molten CaCl2. The effect of the content of nickel additives on the morphology of produced silicon was investigated. Large quantities of SiNWs are obtained by the electrochemical reduction of Ni/SiO2 blocks with SiO2 to Ni molar ratio of 20 and 10. Nickel additives repress the growth of irregular branches and promote longitudinal growth of SiNWs. Wire morphologies and surfaces are influenced by the electrolysis temperature. SiNWs become thicker with the increase of the electrolysis temperature. The optimum temperature to prepare single crystal SiNWs with high aspect ratio and extraordinary surface quality seems to be 1173 K. The amorphous layer of the silicon nanowire is thinner compared to the SiNWs obtained from the pure SiO2 pellets. The produced SiNWs show a photoluminescence emission peak at about 758 nm at room temperature. This work demonstrates the potentiality for the electrochemical reduction process to obtain large quantities of SiNWs with high quality.
Graphical Abstract
Silicon nanowires (SiNWs) were prepared by the electrochemical reduction of Ni/SiO2 blocks in molten CaCl2. The effects of the content of nickel additives and the electrolysis temperature on the morphology of produced silicon were investigated. Nickel additives promote the oriented growth of SiNWs and the SiNWs become thicker with the increase of electrolysis temperature.








Similar content being viewed by others
References
Cohen GM, Rooks MJ, Chu JO, Laux SE, Solomon PM, Ott JA, Miller RJ, Haensch W. Nanowire metal-oxide-semiconductor field effect transistor with doped epitaxial contacts for source and drain. Appl Phys Lett. 2007;90(23):233110.
Liu L, Bao XQ. Silicon nanowires fabricated by porous gold thin film assisted chemical etching and their photoelectrochemical properties. Mater Lett. 2014;125(12):28.
Fang C, Agarwal A, Widjaja E, Garland MV, Wong SM, Linn L, Khalid NM, Salim SM, Balasubramanian N. Metallization of silicon nanowires and SERS response from a single metallized nanowire. Chem Mater. 2009;21(15):3542.
Laïk B, Eude L, Pereira-Ramos JP, Cojocaru CS, Pribat D, Rouvière E. Silicon nanowires as negative electrode for lithium-ion microbatteries. Electrochim Acta. 2008;53(17):5528.
Chan CK, Peng H, Liu G, McIlwrath K, Zhang XF, Huggins RA, Cui Y. High-performance lithium battery anodes using silicon nanowires. Nat Nano. 2008;3(1):31.
Hofmann S, Ducati C, Neill RJ, Piscanec S, Ferrari AC, Geng J, Dunin-Borkowski RE, Robertson J. Gold catalyzed growth of silicon nanowires by plasma enhanced chemical vapor deposition. J Appl Phys. 2003;94(9):6005.
Yao Y, Li F, Lee ST. Oriented silicon nanowires on silicon substrates from oxide-assisted growth and gold catalysts. Chem Phys Lett. 2005;406(4–6):381.
Zhang HZ, Yu DP, Ding Y, Bai ZG, Hang QL, Feng SQ. Dependence of the silicon nanowire diameter on ambient pressure. Appl Phys Lett. 1998;73(23):3396.
Hanrath T, Korgel BA. Supercritical fluid–liquid–solid (SFLS) synthesis of Si and Ge nanowires seeded by colloidal metal nanocrystals. Adv Mater. 2003;15(5):437.
Li Z, Chen Y, Li X, Kamins TI, Nauka K, Williams RS. Sequence-specific label-free DNA sensors based on silicon nanowires. Nano Lett. 2004;4(2):245.
Nohira T, Yasuda K, Ito Y. Pinpoint and bulk electrochemical reduction of insulating silicon dioxide to silicon. Nat Mater. 2003;2(6):397.
Jin X, Gao P, Wang D, Hu X, Chen GZ. Electrochemical preparation of silicon and its alloys from solid oxides in molten calcium chloride. Angew Chem. 2004;116(6):751.
Yang J, Lu S, Kan S, Zhang X, Du J. Electrochemical preparation of silicon nanowires from nanometre silica in molten calcium chloride. Chem Commun. 2009;22(22):3273.
Nishimura Y, Nohira T, Kobayashi K, Hagiwara R. Formation of Si nanowires by direct electrolytic reduction of porous SiO2 pellets in molten CaCl2. J Electrochem Soc. 2011;158(6):E55.
Zhao J, Li J, Ying P, Zhang W, Meng L, Li C. Facile synthesis of freestanding Si nanowire arrays by one-step template-free electro-deoxidation of SiO2 in a molten salt. Chem Commun. 2013;49(40):4477.
Yasuda K, Nohira T, Takahashi K, Hagiwara R, Ogata YH. Electrolytic reduction of a powder-molded SiO2 pellet in mlten CaCl2 and aceleration of reduction by Si addition to the pellet. J Electrochem Soc. 2005;152(12):D232.
Zhang YF, Tang YH, Peng HY, Wang N, Lee CS, Bello I, Lee ST. Diameter modification of silicon nanowires by ambient gas. Appl Phys Lett. 1999;75(13):1842.
Liu SM, Yang Y, Sato S, Kimura K. Enhanced photoluminescence from Si nano-organosols by functionalization with alkenes and their size evolution. Chem Mater. 2006;18(3):637.
Acknowledgments
This study was financially supported by the National Natural Science Foundation of China (No. 51404032 and No. 51504032) and the National High Technology Research and Development Program of China (No. 2013AA050904).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Fang, S., Wang, H., Yang, JY. et al. Electrochemical preparation of silicon nanowires from porous Ni/SiO2 blocks in molten CaCl2. Rare Met. 38, 776–782 (2019). https://doi.org/10.1007/s12598-016-0742-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12598-016-0742-3