Abstract
This study investigates the molluscan fauna of the South Azorean Seamount Chain (SASC), which comprises several seamounts culminating in 300–1600 m depth, separated by distances less than 200 km. Material was collected mainly by dredging and comprises mostly empty shells. A total of over 111,000 shells representing at least 439 species (409 identified) was collected. Larval development was inferred from protoconch morphology, and the assemblage comprises species with planktotrophic larvae, with non-feeding planktonic larva, and with lecithotrophic larvae with direct development. The direct developers are more prevalent among species endemic to the SASC in the upper bathyal part (300–800 m) of the seamounts, whereas most planktotrophic species are shared with the Lusitanian seamounts and/or the European mainland. Nevertheless, there are notable exceptions to this trend, where species with non-planktotrophic larvae are also widespread, and a large proportion of the species with non-feeding planktonic larvae are shared with Eastern and/or Western Atlantic. Level of endemism of Mollusca is high within the SASC (22.5% overall, 35.8% considering only the interval < 800 m) and even higher (32.6% overall) when considering together the SASC and the Azores. The generic composition and large set of overlapping fauna suggest a strong relation to the temperate Eastern Atlantic, whereas only 19% of the species are shared with the Western Atlantic.
Similar content being viewed by others
Introduction
The geographic range and the local abundance of animals are the result of a suite of factors which first involve their ability to arrive, and next the ability to persist and form viable populations (Barroso et al. 2022; Jablonski 1986; Pechenik 1999; Strathmann 1985). The demands of each of those steps may involve contradictory factors.
In a first approach, we could predict that the more remotely a site is located, the less easily it can be colonized by species which do not spend a long time in the plankton as drifting larvae or propagules. Here, however, a second element has to be considered. For a species having succeeded in establishing a reproductive population in a very isolated site, the long planktonic larval development leads to the loss in the plankton of most of the progeny and constitutes a selective disadvantage compared to species which have a direct development without a planktonic stage. Direct development, if it makes colonization a rare event, is also a favorable factor for the sustainability of the founding population (Johannesson 1988). Planktonic larval dispersal is effective in recolonizing the sector in the next generation only if the colonizable environment is large enough. Below a critical threshold of geographical extent, the species can only be maintained by recurrent recolonization by larvae from the continent (we then speak of pseudopopulations, Bouchet and Taviani 1992). There is therefore a priori a contradiction between the prediction of an easier arrival of species with planktotrophic larval development, and that of a more efficient colonization by species with non-planktotrophic development.
The South Azorean Seamount Chain (SASC) comprises a varied set in terms of age, dimensions, and relative distances to the mainland, making therefore a natural laboratory for testing these aspects of the evolution of marine species. They are isolated by large stretches of deep oceans, with distances to the mainland in the order of 1500 km, and to the Azores of ca 600 km. The very large Great Meteor seamount forms a flat plateau of nearly 2000 km2 culminating at ca 300 m depth, surrounded by very steep cliffs which reach down to the surrounding abyssal plain. The other seamounts have a smaller plateau (Irving with about 750 km2, Atlantis and Hyères with about 350 km2) or a summit deeper than 600 m like Plato. The SASC originated from the activity of the long-lived Azores mantle plume beneath the mid-Atlantic oceanic lithosphere (Ribeiro et al. 2017). Great Meteor is not a piece of drifting continental plate, as had been once suggested (Fleischer et al. 1970), and the flattened plateaus of the seamounts are believed to be abrasion surfaces formed near sea-level (Tucholke and Smoot 1990). However, determining here the age is not straightforward because the seamounts have a complex history of sinking and rejuvenation. The oldest structure in the area is believed to be the deep Cruiser plateau at the base of Irving/Cruiser seamount (50–76 million years) now sunken in more than 2000 m. Following the reconstructed plate movement, the seamounts have younger ages (Plato: 40 million years; Atlantis: 20 million years; Irving: 17 million years; Meteor 11–16 million years) along a back-and-forth track in the area (Tucholke & Smoot 1990). The seamounts are intra-plate structures, which means that their distance from the mainland or from each other has remained unchanged since their formation. Therefore, dispersal is the sole process for benthic life to colonize them.
The sedimentary bottoms on the seamounts are characterized by the lack of any terrigenous input of fine sediments (von Rad 1974), contrary to what occurs along continental margins. All new sediment, apart from the erosion of the geological structure itself, must originate from the production of bioclasts by pelagic and benthic organisms. This is a slow process and allows time for most of the fine particles to be winnowed away. The winnowing and the rugged topography also result in the availability of large rock surfaces in the 200–600 m depth interval, which is usually clad with sediments along the continental margin.
This study aims to investigate the molluscan fauna of the South Azorean Seamount Chain, assembling a taxonomic checklist as complete as possible with relevant data (type of larval development, trophic group, body size, depth range, geographical distribution) regarding the species. We then will attempt to find general patterns related with those attributes: how does geographical distribution relate with type of larval development? Do species with a direct or generally non-planktonic larval development have a more restricted distribution than those with a planktonic phase? Do endemics form a significant part of bathyal mollusc fauna in the SASC? Are specimen size and depth range correlated with different larval development types? Is the origin of the malacofauna rather derived from the Eastern Atlantic, the Western Atlantic, or elsewhere?
Material and methods
Sample collection and processing
Material from the North Atlantic seamounts was collected during the French Seamount 2 expedition and the German M151 and POS397 expeditions (Fig. 1). Seamount 2 was conducted in January/February 1993 by SG with R/V Le Suroit and visited the Great Meteor, Hyères, Irving, Plato, Atlantis, Tyro, and Antialtair seamounts (Gofas 1993; material from the latter two seamounts still unsorted and not taken into account in this study), of which 100 operations on Great Meteor, Hyères, Irving, Plato, and Atlantis (17 beam trawls, 74 dredge haul, 8 epibenthic sleds, and one suprabenthic sled) are taken into account in this study. M151 visited Great and Little Meteor seamounts, Atlantis, and several small seamounts in the vicinity of the Azores Eastern group of islands in October 2018 by AF and LH (Frank 2018), collecting box core and van Veen samples, and a ROV with various devices for sampling, of which 12 are studied here; the ROV sediment samples were small but focussed on specific prospective habitats near corals, octocorals and sponges. The POS397 expedition only visited the upper plateau of Great Meteor in March 2010 and this study incorporates 21 samples collected with a small Shipek grab (up to ca 1 L of sediment), aimed to evaluate the sediment composition (George 2010).
Scope of the present study in the South Azorean Seamount Chain (Seamount 2, M151, and POS397 expeditions) and the Azores eastern group (M151). The grey arrows indicate the overall direction of near-surface water circulation, redrawn from a near-surface velocity model in Johnson and Stevens (2000)
The coarse fractions were mostly sorted on board to phyla and later sorted to species level. Samples of the finer fractions were preserved on board, sieved on 5, 2, 1, and 0.5 mm sieves, and sorted for separating specimens under a stereomicroscope. Particular attention was given to cleaning the collecting gear when proceeding from one seamount to another, in order to avoid contaminations. The nets were thoroughly washed on deck during transit between seamounts, and sieves were stained in methylene blue.
Identification of specimens and assembly of the data matrix
Publications covering a large area of the North Atlantic, as well as comparative material from museum collections (mostly MNHN, Paris), were used for species identification. Important works are a series of monographs on North-West Atlantic deep-sea gastropods (Bouchet and Warén 1980, 1985, 1986, 1993; Warén 1989a, 1991, 1992, 1993, 1996a; Warén and Bouchet 1991; Warén and Gofas 1997) and deep-sea bivalves (Allen and Morgan 1981; Allen and Turner 1974; Krylova 2006; Salas 1996; Sanders and Allen 1973, 1977; Warén 1989b) in addition to the works on CANCAP Molluscs (Dijkstra and Goud 2002; van Aartsen et al. 1998, 2000; van der Linden 1995; 1998; Verhecken 2007). Species which are not named at the species level are denoted by “n. sp.” (or “n. gen. n. sp.” if appropriate) when they are under study and recognized as new taxa pending formal description, and those are taken into account in the assessment of endemism. Other species denoted as simply “sp.” are only recognized as a morphospecies for which we cannot venture a species name, and are excluded from those estimates.
Species occurrences and numbers were taken from the published articles for those groups already studied: Anatoma (Hoffman et al. 2021), Clelandella (Gofas 2005), Calliostoma (Gofas and Hoffman 2020), Skeneidae (Hoffman et al. 2020a; Rubio et al. 2015, 2019); Seguenziidae (Hoffman et al. 2020b); Kaiparapelta (Warén and Gofas 1996); Fissurellidae (Hoffman and Freiwald 2023); Eulimidae (Hoffman and Freiwald 2020); Papuliscala (Hoffman et al. 2020c), Rissoidae (Gofas 2007; Hoffman and Freiwald 2021); Trituba (Gofas 2003); Cerithiopsidae and Newtoniellidae (Gofas et al. 2023); Haloceras (Gofas 2018); Pedicularia (Lorenz 2009); Tonnoidea (Gofas and Beu 2003); Muricidae (Houart 1996; Oliverio and Gofas 2006); Fasciolariidae (Gofas 2000); Columbellidae (Gofas et al. 2019); Pyramidellidae (Peñas and Rolán 1999); Phyllidiidae (Valdés and Ortea 1996); Pectinidae (Dijkstra and Gofas 2004); Dacrydium (Salas and Gofas 1997). For other groups, the identified specimens were counted from the original collection. Families Architectonicidae and Mathildidae are recorded at the family level only, because the material was lost when sent on loan for study (estimated 10–15 species, all with planktotrophic larval development). Additional occurrences of identified species found in the collection, and which had not been taken into account in the published accounts, were added. The study concentrated on benthic species only and therefore all records of planktonic pteropods, heteropods, and species in Janthina were removed from the data set. The occurrences of the species in each sampling location are detailed in supplementary Table S1.
Attributes of the species
Each species was qualified with an assessment of its type of larval development, feeding type, size range, depth range, and known biogeographic distribution. The list of species with their attributes is given in Table S2.
Larval development
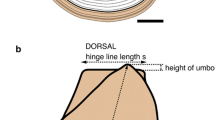
Molluscs are the only animals in which clues to the mode of larval development can be found in the adult (Jablonski and Lutz 1980) The larval shell of gastropods (called protoconch) and that of bivalves (called prodissoconch), is usually retained in the adult shell and, in small-sized specimens, it is usually pristine. The protoconch is interpreted as indicating planktotrophic development when it is multispiral (typically > 2 whorls), but above all when there is a differentiated protoconch 1 and protoconch 2 (Fig. 2a, b), and as non-planktotrophic when it is paucispiral (typically 1–1½ whorl with only one unit) or when, albeit multispiral, there is no discontinuity between the first protoconch whorl and the following (see Bouchet and Warén 1993: 596, and Gofas 2003). Among the non-planktotrophic larval type, further distinction is made between planktonic but supposedly non-feeding (but see Jaeckle and Manahan 1989) larvae, typical of, e.g., Vetigastropoda, denoted by a small larval shell with generally a well-defined outer lip separating it from the teleoconch (Fig. 2f, g), and lecithotrophic development where the planktonic stage is either completely lacking (the juveniles count on yolk for intracapsular development and hatch as benthic juveniles), or the larvae hatch as pelagic late veligers swimming for a very short time before settlement. Whether a particular species can shift between lecithotrophic with or without a short planktonic phase (poecilogony) has been debated (Bouchet 1989) but seems demonstrated in some cases (e.g., Warén 1996b). The protoconch morphology does not allow to detect those variations in the timing of hatching and settlement, and all developmental types inferred to lack a substantial planktonic stage will be scored as “direct.” In those species lacking a substantial planktonic stage, the transition from protoconch to teleoconch may be gradual (Fig. 2i), or marked by a distinct suture and a drastic change in sculpture (Fig. 2j, k).
Examples of protoconchs; a–e denoting planktotrophic development; a–b Halgyrineum louisae (Lewis, 1974), Atlantis, SMT2 DW274; c–e: Spondylus gussonii (Costa, 1830), Atlantis, SMT2 DW274; f–h denoting non-feeding planktonic development; f Danilia affinis Dautzenberg & Fischer, 1896, Atlantis, SMT2 DW274; g Propilidium exiguum (Thompson, 1844), Atlantis, SMT2 DW274; h Tindaria sericea (Jeffreys, 1876), off Morocco 1378 m, BALGIM CP95; i–l denoting direct development; i Cerithiella metula (Lovén, 1846), Atlantis, SMT2 DW261; j Haloceras meteoricum Gofas, 2018, Great Meteor, SMT2 DW152; k Fusinus meteoris Gofas, 2000, Great Meteor, SMT2 DW152; l Dacrydium dauvini Salas & Gofas, 1997, Atlantis, SMT2 DW274. Maximum height for a 2.75 mm; for c 2.85 mm; for k 1.55 mm; scale bars for the other figures 100 µm, small arrows denote protoconch 1-protoconch 2 (or prodissoconch 1-prodissoconch 2) limit, large arrowheads the protoconch-teleoconch (or prodissoconch-dissoconch) limit
Among bivalves, similar patterns are found but without coiling; planktotrophic species have a differentiated prodissoconch 1 and prodissoconch 2 (Fig. 2c–e), whereas species with non-feeding larvae have a small, continuous prodissoconch (Fig. 2h). Bivalves with a direct development often brood their larvae (Salas and Gofas 1997) and those have a prodissoconch without internal discontinuity, often with a rim at the prodissoconch/dissoconch limit (Fig. 2l). In scaphopods, larval development is poorly known but, in the few species where data are available, the larvae are non-feeding even if they go through a short planktonic phase (Engeser et al. 1993).
The inference of larval development is not always straightforward. The small paucispiral larval shell of Vetigastropoda is generally interpreted as denoting a short non-feeding planktonic stage, but brooding of larvae has been documented in at least one species (Williams et al. 2020). Among heterobranchs, there is no obvious protoconch 1 and 2, and the significance of protoconchs has been debated. In the family Pyramidellidae, the axis of the protoconch is at an angle with that of the teleoconch, and whereas the multispiral protoconchs with an apparent spire (type A and B) are interpreted as planktotrophic, those with a concealed spire do not allow a whorl count and are ambiguous (Robertson 2012). The heterobranch genus Acteocina features both species with planktotrophic and non-planktotrophic larvae, the difference in protoconch morphology being only a small difference in the number of whorls (Mikkelsen and Mikkelsen 1984). No larva of any septibranch has been described (Morton and Machado 2019). Clues from other anomalodesmatan bivalves suggest that both brooded embryos and short non-feeding pelagic larvae are possible, but brooded larvae were never reported in the large array of septibranchs examined anatomically (Allen and Turner 1974; Allen and Morgan 1981) so that a short non-feeding planktonic larva is the most probable condition in deep-sea septibranchs. Nonetheless, larval development for those species is here scored as “unknown.”
Size range
Specimens were measured with a precision of 0.02 mm using an ocular micrometer under the stereomicroscope for the whole specimens. The species were distributed in size classes following Bouchet et al. (2002) with the following intervals: < 1.8 mm, 1.9–4.1 mm, 4.2–8.7 mm, 8.8–18.9 mm, 19.0–40.9 mm, 41–88 mm, 88–199 mm, > 200 mm (selected because they have intervals that are equivalent in a logarithm transformation). The first three categories fall within the size class known as “micromolluscs” (Sasaki 2008). Most of the species considered here correspond to macrofauna (0.5–50 mm, Watling 2019), very few to megafauna.
Trophic groups
Species were assigned to trophic groups, mainly inferred from the higher taxa to which they belong when this attribute is known to be homogeneous. All the samples are located beyond the photic zone, therefore without herbivores. Four categories were considered: filter-feeders, deposit-feeders, predators, and specialized ectoparasites. Grazers on sessile invertebrates (e.g., Diodora, Calliostoma, Epitonium) were counted as predators, whereas small species more or less attached to a host (e.g., Pyramidellidae, Eulimidae, Cerithiopsidae, Montacutidae) were counted as ectoparasites (called “micropredators” by Poulin 2011), but the divide between this and the “predators” group is blurry. Taxa for which data are not straightforward were not scored.
Depth range
Depth range was qualified as upper bathyal (i.e., characteristic of the upper part of the seamounts) or lower bathyal according to the prevalent occurrence of the species in the upper bathyal (< 800 m) or lower bathyal (> 800 m) sample groups. The divide between upper bathyal and lower bathyal samples is taken at the 800 m isobath, proposed as the boundary between upper and lower bathyal provinces by Watling et al. (2013). Species were scored as upper bathyal where their abundance in the upper bathyal samples was more than twice their abundance in the lower bathyal samples, and vice-versa; those with balanced abundance were not scored. The 800 m isobath corresponding to Watling et al. zonation was downloaded from the Marine Regions website (Flanders Marine Institute 2023), and the 2000 m isobath (containing all of our samples) from Natural Earth (2023). These were plotted in a GIS in order to assess the surfaces of seabed involved in each depth range (less than 800 m and 800–2000 m).
Geographical distribution
Species were scored for their presence/absence on each of the five seamounts. Data of occurrence of the species outside the SASC were derived from the relevant literature, in some cases completed with our own data (mainly BALGIM 1984 and SEAMOUNT1 1987 cruises in MNHN, Paris, and, among others, a large set of bottom sediment samples from German and Dutch research cruises in upper bathyal Cold Water Coral (CWC) habitats in NW Morocco (Moundforce 2004), Lusitanian Seamounts, Galicia Bank and Bay of Biscay (VH97), Rockall Bank (M61-1; M61-3; POS316; Moundforce 2004, HERMES 2005, HERMES 2006), and Hatton Bank (HERMES 2008)). For the Eastern Atlantic continental slope, here understood as the slopes of Morocco, the Iberian Peninsula, and Bay of Biscay, a wealth of data are available, and we mainly used records (excluding the shallow-water fauna) for “Demarcación Noratlántica” and “Demarcación Suratlántica” in the Spanish checklist (Gofas et al. 2017) which takes into account all previous records to that date; those were completed for Portugal and Morocco using Bouchet and Warén (1980, 1985, 1986, 1993) for gastropods and Allen (2008) for bivalves. For the Azores, we used Dautzenberg and Fischer (1896, 1897), Bouchet and Warén (1980, 1985, 1986, 1993) and original results from cruise M151. Occurrences in the Canaries are taken from “Demarcación Canaria” in the Spanish checklist (Gofas et al. 2017) excluding the shallow-water fauna, and completed with Ortega and Gofas (2019); in Madeira from Segers et al. (2009); on the Lusitanian seamount mainly on the BIAS atlas (Beck et al. 2006); on Galicia Bank, on Gofas et al. (2021); on Rockall and Hatton banks mainly on our original data and Hoffman et al. (2011) and references therein.
There is no obvious literature source for the distribution of the deep-sea fauna of the Western Atlantic and there we used the Malacolog database (Rosenberg 2009), downloading faunal lists for Florida, the Carolinas, Georgia, Cuba and Puerto Rico as a representation, and retaining from these only the species whose depth ranges are recorded between 300 and 1500 m.
Searching for trends
The type of larval development was matched against depth intervals of the samples, size classes, and biogeographical attributes. The abundance of each species was plotted against the type of larval development, in each of the depth ranges < 800 m and > 800 m (the divide between Upper and Lower bathyal zones according to Watling et al. 2013).
Regarding distribution, species were rated according to the number of seamounts in the SASC where recorded, and this in turn was matched against the type of larval development. Endemism is defined as the relation of a species to an area. The percentage of endemic species was investigated for different geographical scopes: single seamounts, the SASC as a whole, the central North Atlantic (SASC + the Azores excluding coastal fauna), and broader areas in the North Atlantic.
Affinity between samples
We only retained for this analysis the samples in which at least 200 specimens were recovered. Affinity between samples was assessed based on the abundance data of the species, using the software PRIMER (v. 6, Clarke and Warwick 1994). The data were transformed by applying the fourth root (executed by default in PRIMER v.6), which reduces the distortion brought by the very abundant species on the similarity between two samples. The Bray–Curtis similarity index was calculated from this transformed matrix.
Similarity was visualized with a dendrogram (CLUSTER function in Primer6, using UPGMA algorithm) and nMDS (Non-Metric Multidimensional Scaling). Dendrogram nodes are distributed according to an axis indicating percentage of similarity between samples. In MDS, the points (samples) are represented graphically with a distance reflecting the similarity between the samples. In the nMDS, a stress coefficient measures the discrepancies between the resulting distances (value of less than 0.1 suggest a very reliable representation, values above 0.3 suggest an almost arbitrary arrangement; Clarke and Warwick 1994).
A SIMPER (SIMilarity PERcentage) analysis was performed in order to know the contribution of the species in the similarity/dissimilarity within and between the groups of samples which resulted from the clustering. The ANOSIM test, through a RANOSIM was applied to assess the significance of the differences between groups (significant with RANOSIM value approaching 1 or − 1, not significant when RANOSIM value near 0). The analysis was performed on two datasets, one in which samples with less than 200 specimens in the catch were excluded (this reduced dataset representing 95% of the total catch), and another only excluding samples with less than 10 specimens. The contribution of the species to the similarity/dissimilarity within and between groups of samples was assessed using the SIMPER (SIMilarity PERcentage) routine of Primer6.
Affinities with the biogeographical context
For this analysis, we considered not only the occurrences of the seamount species, but also representative lists of deep-sea species which were not found on the SASC (same sources as for species shared with the SASC). We scored presence/absence for the Azores, Madeira, the Canaries, the Lusitanian seamounts (Gorringe, Ampère, Seine, Josephine, Coral Patch), Galicia Bank, and the Rockall and Hatton banks in the Eastern North Atlantic, for Florida, Carolinas, Georgia, Cuba, and Puerto Rico in the Western North Atlantic. The list of species with their score in these areas is given in supplementary Table S3.
The method used to identify the biogeographic affinity between the SASC and its geographical context in the Atlantic is based on Olivero et al. (2013) and the “RMacoqui 1.0” software (http://rmacoqui.r-forge.r-project.org/). Geographic units are classified hierarchically according to the presence/absence of the species, using the Baroni-Urbani and Buser similarity index and the UPGMA algorithm. The G-tests of independence (Sokal and Rohlf 1981) were used to determine significant boundaries for the resulting geographical units between classification clusters. For further information regarding the methodology, we refer to Olivero et al. (2013).
Results
A total of over 111,000 specimens were obtained from the area of interest including Meteor, Hyères, Plato, Irving, and Atlantis seamounts (ca. 89,000 in Seamount 2 samples, 22,000 in M151 and POS297). The total of 133 operations resulted in very unequal yields (supplementary Table S1). The beam trawls performed poorly, most of them with less than 10 mollusc specimens. The 15 most successful operations (5 dredge hauls on Hyères, 5 on Atlantis, 2 on Plato, 2 on Irving, and only one on Meteor) obtained 1000 to 10,000 specimens each, whereas nearly half of the operations obtained less than 200 specimens and 15, less than 10. Most of the material consisted of empty shells, whereas only 123 species were represented by 1952 live-taken specimens (1.7% of the total catch) in the Seamount 2 material and 20 in the M151 and POS397 material. The beam trawls, despite the paucity of their overall catch, accounted for many of the live-taken megafaunal species (e.g., five of the 17 live-taken Ranella olearium (Linnaeus, 1758) and 16 of the 38 live-taken Tritonoranella ranelloides (Reeve, 1844), whereas the grab samples only exceptionally would yield live specimens. The small Shipek grab samples taken during POS397 yielded usually less than 300 shells and the ROV sediment samples taken during M151 typically less than 1000 shells. Box core and van Veen samples taken during M151 provided higher numbers of molluscs; most box core samples contained more than 1000 individuals.
A total of 437 taxa could be recognized (Table S1), of which 409 were identified to the species level (this including 19 species, mostly in the superfamily Conoidea, recognized as undescribed and currently pending a formal description). Another 28 were recognized as putative morphospecies for which an identification could not be ventured.
Abundance of species is utterly unbalanced (Fig. 3), with the 20 most abundant species accounting for half of the individuals. At the other end, there are 96 species represented by less than 10 specimens and 20 represented by only one specimen. The very abundant species are all micromolluscs, with the megafauna accounting for a very small part (360, i.e., 0.3%) of the total specimens and shells count.
Plot of abundance of the 100 most abundant species on the SASC, ranked in decreasing values. Basilissopsis athenae Hoffman, Gofas & Freiwald, 2020, Gibberula vignali (Dautzenberg & Fischer, 1896), Gofasia atlantidis Gofas, 2007, Alvania adiaphoros Bouchet & Warén, 1993, Cirsonella gaudryi (Dautzenberg & Fischer, 1896), Porosalvania solidula Gofas, 2007, Porosalvania profundior Gofas, 2007, Pedicularia sicula Swainson, 1840, Asperarca nodulosa (Müller, 1776), Limopsis minuta (Philippi, 1836), Alvania elenae Gofas, 2007, Amphissa acutecostata (Philippi, 1844), Parvamussium intuslaeve Dijkstra & Gofas, 2004, and Alvania microtuberculata Gofas, 2007, are represented by more than 2000 shells, and half of these species are endemic to the SASC (denoted by an asterisk)
Size classes
Most of the species and specimens found on the seamounts are small. As shown in Fig. 4, planktotrophic larval development is not recorded among the tiniest (< 1.8 mm) molluscs, and is quantitatively unimportant in all micromolluscs (36 out of 134 species in the 4.1 size class but these 36 species account for only 2% of the total individuals). Planktotrophic larval development is more prevalent in the 8.7 mm size class and above (14 of 25 species and half of the specimens in the 40.9 + 88 size classes). The megafauna (the last size class) comprises only four species and 366 specimens.
Trophic groups
Predators, most of them conoidean neogastropods, are the most numerous in the species count, followed by ectoparasites. However, those groups are minorities in the specimen count, where detritus feeders account for half of the total. Filter feeders are few, both as species and as individuals (Fig. 5).
Species-level endemism
Patterns of endemism are detailed in Table 1, showing that species are seldom found on a single seamount. There are 93 species (22.5% of the 409 identified) which were found on one or more of the SASC seamounts and have never been reported elsewhere (Table 1; Figs. 6 and 7). Most of them were described in this century from this material, or belong to the species under study; the exceptions are Trituba superstes (Bouchet & Fechter, 1981), T. anelpistos (Bouchet & Fechter, 1981), and Cuspidaria meteoris Krylova, 2006, described from material collected by the German cruise 9C of R/V Meteor in July 1967, and Calliostoma heugteni Vilvens & Swinnen, 2003, described from a shell of unknown origin. Among these, 62 (35.8%) of the 173 identified species scored as upper bathyal but only 15 (12.0%) of the 125 species scored as lower bathyal are only found on the SASC. A further 50 species identified in this study on the SASC are also reported from the Azores, but not elsewhere.
Examples of species which were only found on the SASC and are presumably endemic; a Asthelys hyeresensis Hoffman, Gofas & Freiwald, 2020, Hyères, SMT2 DW197 (4.5 mm); b Basilissopsis athenae Hoffman, Gofas & Freiwald, 2020, Hyères, SMT2 DW182 (2.2 mm); c Clelandella perforata Gofas, 2005, Atlantis, SMT2 DW254 (6.5 mm); d Parviturbo seamountensis Rubio, Rolán & Gofas, 2015, Great Meteor, SMT2 DW152 (1.75 mm); e Calliostoma heugteni Vilvens & Swinnen, 2003, Atlantis, SMT2 CP257, H 6.0 mm; f Calliostoma cyrtoida Gofas & Hoffman, 2020 (holotype), Hyères, SMT2 DW200 H 6.7 mm; g Calliostoma freiwaldi Gofas & Hoffman, 2020, Hyères, SMT2 DW182 (13 mm); h Cerithiella seilaeformis Gofas, Freiwald & Hoffman, 2023, Hyères, MT2/DW182 (4.0 mm); i Krachia meteoris Gofas, Freiwald & Hoffman, 2023, Plato, SMT2 DW240 (2.7 mm); j Haloceras meteoricum Gofas, 2018, Great Meteor, SMT2 DW152 (2.2 mm); k Parvamussium intuslaeve Dijkstra & Gofas, 2004 (holotype), Great Meteor, SMT2 DW143 (3.9 mm). Species a to d have non-feeding planktonic larvae, h to k are inferred to be direct developers
Examples of species of the superfamily Rissooidea described by Gofas (2007) as endemic to the SASC, all direct developers; a Alvania elenae, Great Meteor SMT2 DW152 (2.6 mm); b Alvania hyerensis, Hyères, SMT2 DW182 (3.0 mm); c Alvania suroiti, Hyères, SMT2 DW182 (2.6 mm); d Alvania microtuberculata, Atlantis, SMT2 DW255 (2.1 mm); e Gofasia atlantidis, Atlantis, SMT2 DW255 (1.8 mm); f Gofasia obtusellaeformis, Atlantis, SMT2 DW255 (1.5 mm); g Porosalvania solidula, Great Meteor, SMT2 DW152 (3.1 mm); h Porosalvania angulifera, Great Meteor, SMT2 DW143 (2.1 mm); i Porosalvania vixplicata, Atlantis, SMT2 DW263 (2.3 mm); j Porosalvania profundior, Atlantis, SMT2 DW261 (3.0 mm); k Rissoina meteoris, Great Meteor, SMT2 DW143 (6.2 mm); l Schwartziella peregrina, Great Meteor, SMT2 DW143 (6.2 mm)
Levels of endemism drop drastically when only one particular seamount is considered. Only Trituba superstes, T. incredita Gofas, 2003, and one undescribed conoidean were found only on Meteor. On Atlantis, we find the highest figure but it still represents only 6.4% of the species present there. The rissoids Gofasia atlantidis Gofas, 2007, and G. obtusellaeformis Gofas, 2007, totalize respectively 3760 and 1085 specimens were found nowhere else and are probably genuinely endemic of Atlantis Seamount, but other species found as very few specimens (e.g., the three specimens of the nudibranch Reticulidia gofasi Valdés & Ortea, 1996) are likely to be found on other seamounts.
There are several local radiations, believed to be endemic of the SASC + Azores area, in the genera Trituba, Krachia, Papuliscala, in the family Rissoidae (Fig. 7), and in a possible new genus in the conoidean family Borsoniidae.
Relating larval development with geographic range
The number of species found in the SASC (spanning from only one to all five seamounts) is quite balanced for each category of larval development (Fig. 8a). From the quantitative point of view, the species represented in all five areas totalize a considerably higher number of specimens (Fig. 8b), i.e., the more widespread species are also much more abundant.
Patterns of larval development for molluscan species recorded in the SASC, according to the number of seamounts on which they were collected; a all species (species count); b all species (specimen count); c upper bathyal group (species count); d upper bathyal group (specimen count); e lower bathyal group (species count); f lower bathyal group (specimen count)
There is a tendency for the species with direct development being more abundant in the upper bathyal group of samples. In this respect, the prevalence of direct developers (both qualitatively, Fig. 8c, and quantitatively, Fig. 8d) among the upper bathyal species is considerably more among the restricted species (1–3 seamounts) than among the widespread ones, whereas the planktotrophic species are most widespread ones. The trend is not so apparent in the lower bathyal group, where planktonic larvae (both planktotrophic and non-feeding) are important both in the widespread and in the restricted species, and the increase of species with direct development among the restricted species is not marked (Fig. 8e). Quantitatively, the direct developers recorded on one or two seamounts totalize very few specimens (Fig. 8f). In the deep part, all dispersal types do equally, but the abundant species are mostly on all five seamounts.
When only the endemic component (91 species, Table 1, Fig. 9) is considered, the share of direct developers is considerably larger, particularly among the species found only on 1 − 4 seamounts. Species with a short non-feeding planktonic development are also present, their high number in the specimen count for species found on all five seamounts being due to the high numbers of Basilissopsis athenae Hoffman, Gofas & Freiwald, 2020, and, to a lesser extent, Clelandella perforata Gofas, 2005, and Seamountiella dimidia Rubio, Gofas & Rolán, 2019.
If the type of larval development is scored on a broader geographic scale (Fig. 10), we find a markedly higher proportion of species with planktotrophic larval development among the widespread species, shared between the SASC and the Eastern Atlantic continental margin on the one hand, with the Western Atlantic on the other hand.
Patterns of larval development for molluscan species found on the SASC, according to distribution patterns; a species count; b specimen count. SASC + LUS/CAN refers to species found in the SASC and in at least one of the following: Canaries, Madeira, Lusitanian seamounts, Galicia Bank but not on the mainland continental margin; SASC + E Atlantic refers to species found in the SASC and along the continental margin of the Bay of Biscay, Iberian peninsula and/or Morocco; SASC + W Atlantic refers to species found in the SASC and in one of the following: Florida, Georgia, the Carolinas, Cuba, and Puerto Rico
Affinity between samples
Depth appears as a major factor determining the affinity between samples, overriding the location on one or another seamount (Fig. 11a). Twelve Shipek samples from POS397 were all taken on the sandy plateau of the Great Meteor Seamount (cluster 1) with very similar sediment properties and narrow depth range (290–330 m); they cluster with DW143 from the same area and with the samples from the summit of Little Meteor. The deeper samples, predominantly of Hyères seamount, cluster on the right side of the dendrogram. The remaining samples seem to form amorphous clusters from intermediate depths (450–1100 m), with little statistical support and without any comprehensive group displaying a similarity above 50%.
a Dendrogram using abundance data of all mollusc species (fourth root transformed) collected on the SASC (dataset of samples with more than 200 specimens, sample depth (m) in parentheses), and the Bray–Curtis similarity index. Clusters numbered 1 to 3 are discussed in the text; b nMDS plot of the same data. LB refers to lower bathyal samples from exceeding 800 m depth and UB refers to samples less than 800 m
Cluster 1, with an average similarity of 56.5%, is supported by a suite of species, mostly endemic to the SASC and confined to the shallow part of the seamounts (Table 2). Cluster 3 has a low (average 31.0%) similarity and the species pointed out in the ANOSIM comprise several widespread species (Asperarca nodulosa, Spondylus gussonii, Amphissa acutecostata) linked to the habitat of Cold Water Corals. For the large Cluster 2, the ANOSIM yields a list of mostly lower bathyal species which do not appear to characterize a definite assemblage The ANOSIM test yielded a RANOSIM value R = 0.597, p = 0.001 with factor “depth” and an even lower RANOSIM value R = 0.361, p = 0.001 with factor “seamount.” The nMDS plot (Fig. 11b) clearly displays depth as a determining factor, supported by a low-stress value (0.14).
Affinities with the biogeographical context
The similarity between the SASC and the rest of the geographical units analyzed are shown in Fig. 12. The seamount chain is loosely grouped with the eastern Atlantic units and shows significant dissimilarity with respect to the Western Atlantic clusters (G-test = 121.682; p < 0.005). Here, with pooled samples for each seamount and presence/absence data, the similarity between neighboring seamounts (Meteor + Hyères + Irving on the one hand, Plato + Atlantis on the other) is about 80% with strong support, and both clusters are most similar to the Azores.
The number of shared species (Fig. 13) clearly reflects this trend: more than half (62%) of the 409 species identified on the SASC are also found in the Azores, and nearly half are shared with the European mainland and/or the Canaries, Madeira, and the Lusitanian seamounts. Conversely, only 19% are documented in the Western Atlantic.
Discussion
General trends in the type of larval development
Our results show that all types of larval developments are well represented among the Mollusca found on the SASC seamounts, and that the type of larval development matters with respect to distribution. At the local scale (the SASC), there is a trend for species with direct development being more prevalent among the upper bathyal species with a restricted range (1–3 seamounts), which is compliant with predictions. This trend is not so apparent in the lower bathyal group, possibly because the extension of the target areas for propagules is considerably larger. The shallowest part of the seamounts, within the 800 m isobath, is exiguous (Meteor 2050 km2, Hyeres 431 km2, Irving 987 km2, Plato 297 km2, Atlantis 865 km2) compared to the more than 20,000 km2 covered by the 800–2000 m interval around the SASC seamounts and the huge area of bathyal bottoms surrounding the Azores.
This study also clearly shows that the widespread species are represented in larger numbers than the restricted ones (Fig. 8 b, d, f). This can also be stated the other way around: independently of their mode of larval development, the abundant species disperse better than the rare ones because propagule pressure is higher. However, findings restricted to a single seamount only prove endemism when a species is plentiful in one seamount and absent in others sampled with the same effort in the same depth. With this consideration, true one-seamount endemics would be between one and six species per seamount (e. g., Schwartziella peregrina Gofas, 2007, and Trituba superstes (Bouchet & Fechter, 1981) on Meteor, Alvania hyerensis Gofas, 2007, and Porosalvania decipiens Gofas, 2007, on Hyères, Krachia cochleapex Gofas, Freiwald & Hoffman, 2023, and Porosalvania diaphana Gofas, 2007, on Plato, Alvania microtuberculata Gofas, 2007, Gofasia atlantidis and Gofasia obtusellaeformis Gofas, 2007, on Atlantis, also some of the undescribed conoideans on several seamounts).
Rarity has often been correlated to dispersal ability, with good dispersers having diverse range sizes, but poor dispersers tending only to have small to intermediate-sized ranges (Gaston et al. 1997: 16). However, a distinction is to be made between species which are rare because they occur very locally (which is the case of the seamount endemics), and species which are rare because they occur very sporadically over large areas. A textbook example of the latter kind of rarity is Cerithiella candela Fernandes, Garofalo & Pimenta, 2015, a planktotrophic species originally described from seamounts off SE Brazil and found in our material with seven specimens on Great Meteor and Hyères seamounts; at the time of collecting, it could have been taken as a SASC endemic.
Relating larval development with geographic range
The trend for more of the widespread species having planktotrophic development and more of the short-range endemics having direct development is overwhelmingly supported by our data (see Fig. 10). A similar and equally clear trend was found in the shallower (less than 200 m) islands Fernando de Noronha and Atoll das Rocas (off NE Brazil) and in the Vitoria-Trindade Seamount Chain (off SE Brazil) (Leal 2000), where planktotrophs were found to be 39.9–48.3% of the species overall but only 15.8–28.6% of the species endemic to (or rather “only found on”) a particular group of seamounts or islands. Based on the analysis of the 238 species of gastropods present in shallow waters (≤ 200 m) of the Western Atlantic, Barroso et al. (2022) also found that planktotrophic species tend to have the largest distribution range.
This superiority of planktotrophs in becoming widespread must be qualified. Some direct developers also do well (e.g., Eumetula bouvieri (Dautzenberg & Fischer, 1896) and Cerithiella metula (Lovén, 1846) shared with Lusitanian seamounts and the European mainland, Actinotrophon actinophorus (Dall, 1889) shared with the Caribbean). At a more local scale, direct developers such as Alvania adiaphoros Bouchet & Warén, 1993, Onchodia tenuicula Gofas, Freiwald & Hoffman, 2023, and Trophonopsis droueti (Dautzenberg, 1889) are found throughout the SASC and in the Azores, proving that distances in the order of magnitude of 200—600 km are not an obstacle to their dispersal. Alternative modes of dispersal, such as the rafting of detached egg capsules, lecithotrophic larvae, or juveniles, can be hypothesized.
The species with a supposedly short and non-feeding larval stage are surprisingly successful at occupying extensive geographic ranges. Of the 92 species of Vetigastropoda identified on the SASC, only 13 (14.1%) were not found elsewhere and of these, 8 are minute “skeneimorphs” which could easily escape notice. This leaves only Basilissopsis athenae, Asthelys hyeresensis, Parviturbo seamountensis, Clelandella perforata, and Seamountiella dimidia Rubio, Gofas & Rolán, 2019, to be reliably assumed as SASC endemics and there is no species proved to be endemic of one seamount. Conversely, 51 species (55.4%) of those Vetigastropoda, and also all four species of Protobranchia reported on the SASC, are shared with at least one of the mainland margins. Therefore, it could be hypothesized that such larvae, even though considered non-feeding, can survive a long time, maybe weeks, before settling if they do not find promptly an appropriate substrate.
Therefore, any type of larval development could potentially enable a mollusc species to reach the SASC seamounts. The question arises of why some species (e.g., Alvania cimicoides (Forbes, 1844), Tritia recidiva (Martens, 1876), Mitrella canariensis (d’Orbigny, 1840), all recorded from the Azores) which are widespread on the continental margins in the corresponding depth range, have planktotrophic larvae and seem to fulfil all requirements for getting there, do not reach the seamounts. There are entire families usually well represented in mainland faunas which are not found, or are underrepresented on the SASC: among gastropods, Nassariidae and Mitridae are conspicuously absent, Naticidae represented by only one species; among bivalves, large families of endofaunal bivalves such as Cardiidae, Tellinidae, and Semelidae are absent, Veneridae represented by only one species, possibly extinct, on Atlantis. Much of the limiting factors must be related to the lack of a suitable habitat or suitable resources rather than a consequence of dispersal riddle. The same habitat-driven limitation could also explain why do some species which are common on the seamounts (e.g., Latirus rugosissimus Locard, 1897, Tritonoranella ranelloides) and have a good potential for dispersal with their planktotrophic larvae, reach Madeira and the Canaries but not the European mainland where the sediment-clad continental margin may lack the appropriate substrate. The successful species on the SASC are those which find their requirement satisfied on the seamounts. Among them, a significant number of species are known to accompany the cold water coral-related habitats like the spongivore fissurellids (Emarginula tuberculosa, E. christiaensi, Diodora tenuiclathrata), the corallivore Coralliophila richardi, and the byssally attached or sessile epibenthic suspension feeders (Asperarca nodulosa, Spondylus gussonii, Lima marioni). The carnivores are mostly small species, comprising the many conoidean species presumably feeding on the small macrofauna or the meiofauna, and the Eulimidae, Epitoniidae, Pyramidellidae ectoparasitic on larger invertebrates.
That direct developers achieve high number in the seamount summits is an indication of their success, within present time and context. However, geological history has proved that poor dispersal capacity and a restricted range considerably increase extinction risk (Pechenik 1999; Vermeij 1989), when environmental conditions fluctuate. On the long term, some short-range endemics with direct development might go extinct, but there is a large pool of better dispersers to draw from and maintain species richness at its equilibrium level.
Levels of endemism and biogeographic affinity of the SASC molluscan fauna
The level of endemism is high in the SASC (22.5%, rising to 35.8% when considering only the upper bathyal part of the seamounts, and is 32.6% when considering the SASC + the Azores as a whole. Endemism is considerably higher in some taxonomic groups which comprise many direct developers (100% among Trituba; 92% among Rissooidea where only the planktotrophic Benthonella tenella is found throughout the North Atlantic and possibly Alvania funiculata is shared with the Lusitanian seamounts; 75% among Papuliscala). Such levels of endemism surpass the 10% threshold required to define a “province” (Briggs and Bowen 2012). However, genus-level endemism is low (maybe limited to one undescribed conoidean genus in the family Borsoniidae, and to Porosalvania if the single record on Coral Patch Seamount is disregarded), whereas, e.g., in Tropical West Africa, 19 out of 264 bivalve genera (7.2%) are endemic to the province (Caballero-Herrera et al. 2022).
Could this level of endemism result from taxonomic artifacts? Some endemic species seem related to a more widespread mainland species, e.g., Cuspidaria meteoris to C. rostrata (Spengler, 1793), Parvamussium intuslaeve to P. fenestratum (Forbes, 1844), and Krachia meteoris Gofas, Freiwald & Hoffman, 2023, to K. tiara (Monterosato, 1874) and could possibly prove to be local subspecies instead of fully segregated species. Conversely, some non-planktotrophic species were assumed to be shared with the mainland or between seamounts when morphological differentiation was undetected or deemed to fit within intraspecific variation (e.g., Migrogaza rotella (Dall, 1881), Ancistrobasis reticulata, Cerithiella metula), but this assumption is not based on genetic data and there is the possibility of cryptic species. Therefore, one bias compensating the other, we are convinced that the figures given here for levels of endemism are realistic.
The maximum level of endemism is found when considering the entire group of seamounts in the SASC, or the (SASC + Azores) region, whereas levels of endemism are very low (maximum 6.4% on Atlantis) when a particular seamount is considered alone. The same trend was found among the seamounts and island chain off NE and SE Brazil by Leal (2000), where endemisms computed for the islands separately ranged between 5.1% and 16%, when examined as island groups the rate of endemism was 19.2% (23 species) at the northern group and 18.3% at the southern group.
The overall affinity of the SASC clearly lies with temperate Western Europe (Figs. 12, 13). Even among taxa which are currently SASC endemics, several have been shared with Europe in the past: Trituba spp. were present in the European Tertiary (Lozouet 1999), and a species quite similar to Haloceras meteoricum was present in the Italian Pliocene (Bertolaso and Palazzi 2000). Despite acknowledging that most macro- and megafaunal invertebrates (66% across several phyla) are common to the northeastern Atlantic mainland areas, Mironov and Krylova (2006) formulated the hypothesis that the recent Meteor seamounts fauna is mainly a result of a late Pliocene–Pleistocene colonization from the Indo-West Pacific around the southern tip of Africa. We do not find any support to this view, when among Mollusca only a handful of species with teleplanic larvae (Akibumia, Tritonoranella) are shared with the Indo-Pacific.
As a conclusion on levels of endemism, we can see a strong faunal affinity of the SASC with the Western European margin and nearby archipelagos, do not find sensible to give this small, isolated peripheral region the same rank as the larger provinces of continental margins in biogeographic regionalization.
Main Conclusions
-
Species with direct development are locally successful among SASC endemics in the upper bathyal part (300–800 m) of the seamounts, but these are vulnerable in a changing environment.
-
Species with planktotrophic development are mostly found among the larger ones in size and among species shared with other archipelagos, other seamount groups, or the mainland.
-
Species with non-feeding pelagic larval development are prominent in the deeper part (> 800 m) of the SASC, and many are shared with Eastern Atlantic, some also with the Western Atlantic.
-
Widespread species are also commonly the most abundant, but there are exceptions where short-range endemic species locally achieve high abundance.
-
Species with any of the types of larval development can achieve widespread distributions.
-
Level of endemism is high on the SASC (22.5%) or on the SASC + the Azores (32.6%), well above the threshold of 10%, but hardly any genus-level endemism. The affinity with temperate fauna of the Eastern North Atlantic is so clear that segregating a distinct “province” is not found sensible.
References
Allen JA (2008) Bivalvia of the deep Atlantic. Malacologia 50(1):57–173. https://doi.org/10.4002/0076-2997-50.1.57
Allen JA, Morgan RE (1981) The functional morphology of Atlantic deep water species of the families Cuspidariidae and Poromyidae (Bivalvia): an analysis of the evolution of the septibranch condition. Philos Trans R Soc 294:413–546. https://doi.org/10.1098/rstb.1981.0117
Allen JA, Turner JF (1974) On the functional morphology of the family Verticordiidae (Bivalvia) with descriptions of new species from the abyssal Atlantic. Philos Trans R Soc 268(894):401–536. https://doi.org/10.1098/rstb.1974.0038. (pl. 58)
Beck T, Metzger T, Freiwald A (2006) Biodiversity inventorial atlas of macrobenthic seamount animals. Deliverable 25 of the EU-ESF project OASIS (Oceanic seamounts: an integrated study; EVK2-CT-2002–00073, p 126. Available from: https://epic.awi.de/id/eprint/37314/7/OASIS_BIAS.pdf. Accessed 6 June 2023
Barroso CX, da Cruz Lotufo TM, Matos AS, de Macêdo Carneiro PB, Cascon HM (2022) The distribution of marine gastropods is more influenced by larval development than by adult characteristics. Mar Biol 169:83. https://doi.org/10.1007/s00227-022-04069-0
Bertolaso L, Palazzi S (2000) Un probabile rappresentante della famiglia Haloceratidae Warén & Bouchet, 1991 nel Pliocene emiliano. Boll Malacol 34:23–26
Bouchet P (1989) A review of poecilogony in gastropods. J Molluscan Stud 55(1):67–78. https://doi.org/10.1093/mollus/55.1.67
Bouchet P, Lozouet P, Maestrati P, Heros V (2002) Assessing the magnitude of species richness in tropical marine environments: exceptionally high numbers of molluscs at a New Caledonia site. Biol J Linn Soc 75(4):421–436. https://doi.org/10.1046/j.1095-8312.2002.00052.x
Bouchet P, Taviani M (1992) The Mediterranean deep-sea fauna: pseudopopulations of Atlantic species? Deep Sea Res 39(2):168–184. https://doi.org/10.1016/0198-0149(92)90103-Z
Bouchet P, Warén A (1980) Revision of the Northeast Atlantic bathyal and abyssal Turridae (Mollusca, Gastropoda). J Molluscan Stud Supplement 8:1–119. https://doi.org/10.1093/mollus/46.Supplement_8.1
Bouchet P, Warén A (1985) Revision of the Northeast Atlantic bathyal and abyssal Neogastropoda excluding Turridae (Mollusca, Gastropoda). Boll Malacol Supplement 1:123–296. https://doi.org/10.5962/bhl.title.140763
Bouchet P, Warén A (1986) Revision of the Northeast Atlantic bathyal and abyssal Aclidiidae, Eulimidae, Epitoniidae (Mollusca, Gastropoda). Boll Malacol Supplement 2:297–576. https://doi.org/10.5962/bhl.title.140762
Bouchet P, Warén A (1993) Revision of the Northeast Atlantic bathyal and abyssal Mesogastropoda. Boll Malacol Supplement 3:577–840. https://doi.org/10.5962/bhl.title.140732
Briggs JC, Bowen BW (2012) A realignment of marine biogeographic provinces with particular reference to fish distributions. J Biogeogr 39(1):12–30. https://doi.org/10.1111/j.1365-2699.2011.02613.x
Caballero-Herrera JA, Olivero J, von Cosel R, Gofas S (2022) An analytically derived delineation of the West African Coastal Province based on bivalves. Divers Distrib 28(12):2791–2805. https://doi.org/10.1111/ddi.13454
Clarke KR, Warwick RM (1994) Change in marine communities: an approach to statistical analysis and interpretation. Plymouth Marine Laboratory, Bourne Press Ltd., Bournemouth
Dautzenberg P, Fischer H (1896) Dragages effectués par l’Hirondelle et par la Princesse Alice 1888–1895. 1. Mollusques Gastéropodes. Mém Soc Zool France 9: 395–498. pl. 15–22
Dautzenberg P, Fischer H (1897) Dragages effectués par l’Hirondelle et par la Princesse Alice 1888–1896. Mém Soc Zool France 10(139–234):3–7
Dijkstra HH, Gofas S (2004) Pectinoidea (Bivalvia: Propeamussiidae and Pectinidae) from some northeastern Atlantic seamounts. Sarsia 89(1):33–78. https://doi.org/10.1080/00364820410003469
Dijkstra HH, Goud J (2002) Pectinoidea (Bivalvia, Propeamussiidae & Pectinidae) collected during the Dutch CANCAP and MAURITANIA expeditions in the south-eastern region of the North Atlantic Ocean. Basteria 66(1):31–82
Engeser TS, Riedel F, Bandel K (1993) Early ontogenetic shells of recent and fossil Scaphopoda. Scrip Geol Special Issue 2:83–100
Flanders Marine Institute (2023) MarineRegions.org. Available online at www.marineregions.org. Accessed 2023–03–18
Fleischer U, Meyer O, Schaf H (1970) Über den Aufbau der untermeerischen Tafelberge südlich der Azoren anhand eines gravimetrisch-magnetischen Nord-Süd-Profils uber die Große-Meteor-Bank. Meteor Forschungergebn (C) 3:37–47
Frank N (2018) Short Cruise Report. M151. Atlantic thermocline ocean and ecosystems dynamic during natural climate change. Ponta Delgada – Funchal, Portugal. Institut für Umweltphysik, Universität Heidelberg, Germany, unpublished report]. Available from https://www.ldf.uni-hamburg.de/meteor/wochenberichte/wochenberichte-meteor/m149-m152/scr-m151.pdf. Accessed 06 June 2023
Gaston KJ, Blackburn TM, Lawton JH (1997) Interspecific abundance-range size relationships: an appraisal of mechanisms. J Anim Ecol: 579–601. https://doi.org/10.2307/5951
George KH (2010) Abschlussbericht POS397 [DZMB, Senckenberg am Meer, Wilhelmshaven, Germany, unpublished report]
Gofas S (1993) Mission Océanographique SEAMOUNT 2. Compte-rendu et liste des stations. [MNHN, unpublished report]. https://doi.org/10.5281/zenodo.7697124
Gofas S (2000) Four species of the family Fasciolariidae (Gastropoda) from the North Atlantic seamounts. J Conchol 37(1):7–16
Gofas S (2003) An endemic radiation of Trituba (Mollusca, Gastropoda) on the North Atlantic seamounts. Am Malacol Bull 17(1–2):45–63
Gofas S (2005) Geographical differentiation in Clelandella (Gastropoda: Trochidae) in the northeastern Atlantic. J Molluscan Stud 71:133–144. https://doi.org/10.1093/mollus/eyi016
Gofas S (2007) Rissoidae (Mollusca: Gastropoda) from northeast Atlantic seamounts. J Nat Hist 41(13–16):779–885. https://doi.org/10.1080/00222930701298085
Gofas S (2018) A non-planktotrophic haloceratid (Gastropoda) from the Meteor seamount group, central North Atlantic. Iberus 36(2):149–155
Gofas S, Beu A (2003) Tonnoidean Gastropods of the North Atlantic seamounts and the Azores. Am Malacol Bull 17(1–2):91–108
Gofas S, Freiwald A, Hoffman L (2023) New species and new records in Cerithiopsidae and Newtoniellidae (Triphoroidea, Gastropoda) from the Azores and South Azorean Seamount Chain. Iberus 41(1):113–150
Gofas S, Hoffman L (2020) Deep-water Calliostomatidae (Vetigastropoda, Gastropoda) from the South Azorean Seamount Chain. Iberus 38(2):195–211
Gofas S, Luque ÁA, Oliver JD, Templado J, Serrano A (2021) The Mollusca of Galicia Bank (NE Atlantic Ocean). Eur J Taxon 785:1–114. https://doi.org/10.5852/ejt.2021.785.1605
Gofas S, Luque ÁA, Templado J, Salas C (2017) A national checklist of marine Mollusca in Spanish waters. Sci Mar 81 (2): 241–254, and supplementary material. https://doi.org/10.3989/scimar.04543.21A
Gofas S, Luque Á, Urra J (2019) Planktotrophic Columbellidae (Gastropoda) in the northeast Atlantic and the Mediterranean Sea, with description of a new species in the genus Mitrella. Bull Mar Sci 96(1):145–168. https://doi.org/10.5343/bms.2019.0015
Hoffman L, Freiwald A (2020) Bathyal Eulimidae (Gastropoda: Vanikoroidea) from the Azorean seamounts collected during the R/V Meteor Cruise M151 Athena. Misc Malacol 8(6):81–99
Hoffman L, Freiwald A (2021) Bathyal species in Rissoidae (Gastropoda) from Azorean seamounts. Biodivers J 12(4):777–792. https://doi.org/10.31396/Biodiv.Jour.2021.12.4.777.792
Hoffman L, Freiwald A (2023) Species in Fissurellidae (Gastropoda) from the North Atlantic with a focus on the Azorean seamounts. Basteria 87(1):77–96
Hoffman L, Gofas S, Freiwald A (2020a) A large biodiversity of “skeneimorph” (Gastropoda: Vetigastropoda) species from the South Azorean Seamount Chain, with the description of seventeen new species. Iberus supplement 9 [to vol. 38]: 1–82
Hoffman L, Gofas S, Freiwald A (2020b) New and little-known Seguenziidae (Vetigastropoda, Gastropoda) from the South Azorean Seamount Chain. Iberus 38(1):1–18
Hoffman L, Gofas S, Freiwald A (2020c) Ten new species in Papuliscala de Boury, 1911 (Gastropoda, Epitoniidae) from the South Azorean Seamount Chain. Iberus 38(1):29–53
Hoffman L, Gofas S, Freiwald A (2021) The genus Anatoma Woodward, 1859 (Gastropoda, Anatomidae) from Azorean seamounts. Iberus 39(2):139–152
Hoffman L, van Heugten B, Lavaleye MSS (2011) Gastropoda (Mollusca) from the Rockall and Hatton Banks, northeastern Atlantic Ocean. Misc Malacol 4(6):85–118 (5(2): 23-52)
Houart R (1996) Description of new species of Muricidae (Gastropoda) from New Caledonia, the Philippine Islands, the Northeast Atlantic, and West Africa. Apex 11(2):59–75
Jablonski D (1986) Larval ecology and macroevolution in marine invertebrates. Bull Mar Sci 39(2):565–587
Jablonski D, Lutz RA (1980) Molluscan larval shell morphology. Ecological and paleontological applications. In: Rhoads DC, Lutz RA (eds) Skeletal growth of aquatic organisms. Plenum Press, New York, pp 323–377
Johannesson K (1988) The paradox of Rockall: why is a brooding gastropod (Littorina saxatilis) more widespread than one having a planktonic larval dispersal stage (L. littorea)? Mar Biol 99(4):507–513. https://doi.org/10.1007/BF00392558
Jaeckle WB, Manahan DT (1989) Feeding by a “nonfeeding” larva: uptake of dissolved amino acids from seawater by lecithotrophic larvae of the gastropod Haliotis rufescens. Mar Biol. https://doi.org/10.1007/BF00391067
Johnson J, Stevens I (2000) A fine resolution model of the eastern North Atlantic between the Azores, the Canary Islands and the Gibraltar Strait. Deep Sea Res Part I 47:875–899. https://doi.org/10.1016/S0967-0637(99)00073-4
Krylova EM (2006) Bivalves of seamounts of the north-eastern Atlantic. Part 1. In: Mironov AN, Gebruk AV, Southward AJ (eds) Biogeography of the North Atlantic Seamounts. KMK Scientific Press, Moscow, pp 76–95
Leal JH (2000) Endemism and modes of development of marine prosobranch gastropods (Mollusca) from oceanic islands off Brazil. Arquipélago. Life Mar Sci Supplement 2(Part A): 79–87
Lorenz F (2009) Two new Pediculariidae from Hyères Seamount, eastern central Atlantic (Gastropoda: Cypraeoidea). Acta Conchyliorum 10:87–94
Lozouet P (1999) Nouvelles espèces de gastéropodes (Mollusca: Gastropoda) de l’Oligocène et du Miocène inférieur d’Aquitaine (sud-ouest de la France). Partie 2. Cossmanniana 6:1–68
Mikkelsen PS, Mikkelsen PM (1984) Comparison of Acteocina canaliculata (Say, 1826), A. candei (d'Orbigny, 1841) and A. atrata spec. nov. (Gastropoda: Cephalaspidea). Veliger 27(2): 164–192
Mironov AN, Krylova EM (2006) Origin of the fauna of the Meteor Seamounts, north-eastern Atlantic. In: Mironov AN, Gebruk AV, Southward AJ (eds) Biogeography of the North Atlantic Seamounts. KMK Scientific Press, Moscow, pp 22–57
Morton B, Machado FM (2019) Predatory marine bivalves: a review. Adv Mar Biol 84:1–98. https://doi.org/10.1016/bs.amb.2019.10.001
Natural Earth (2023) World map data, available from https://www.naturalearthdata.com/. Accessed 2023–03–18
Oliverio M, Gofas S (2006) Coralliophiline diversity at mid-Atlantic seamounts (Neogastropoda, Muricidae, Coralliophilinae). Bull Mar Sci 79(1):205–230
Olivero J, Marquez AL, Real R (2013) Integrating fuzzy logic and statistics to improve the reliable delimitation of biogeographic regions and transition zones. Syst Biol 62:1–21. https://doi.org/10.1093/sysbio/sys061
Ortega J, Gofas S (2019) The unknown bathyal of the Canaries: new species and new records of deep-sea Mollusca. Zoosystema 41(26):513–551. https://doi.org/10.5252/zoosystema2019v41a26
Pechenik JA (1999) On the advantages and disadvantages of larval stages in benthic marine invertebrate life cycles. Mar Ecol Progr Ser 177:269–297. https://doi.org/10.3354/meps177269
Peñas A, Rolán E (1999) Pyramidellidae (Gastropoda, Heterostropha) de la misión oceanográfica “Seamount 2”. Iberus supplement 5 [to vol. 17]: 151-199. https://doi.org/10.5281/zenodo.4731128
Poulin R (2011) The many roads to parasitism: a tale of convergence. Adv Parasitol 74:1–40. https://doi.org/10.1016/B978-0-12-385897-9.00001-X
von Rad U (1974) Great Meteor and Josephine seamounts (eastern North Atlantic): composition and origin of bioclastic sands, carbonate and pyroclastic rocks. Meteor Forschungsergeb C 19:1–61
Ribeiro LP, Martins S, Hildenbrand A, Madureira P, Mata J (2017) The genetic link between the Azores Archipelago and the Southern Azores Seamount Chain (SASC): the elemental, isotopic and chronological evidences. Lithos 294–295:133–146
Robertson R (2012) Pyramidellid protoconchs, eggs, embryos and larval ecology: an introductory survey. Am Malacol Bull 30(2):219–228. https://doi.org/10.4003/006.030.0201
Rosenberg G (2009) Malacolog 4.1.1. A database of Western Atlantic Marine Mollusca. available from http://www.malacolog.org/. Accessed 06 June 2023
Rubio F, Gofas S, Rolán E (2019) A new genus of small Vetigastropoda from eastern Atlantic Ocean and Indo-Pacific islands and seamounts. Iberus 37(2):249–265
Rubio F, Rolán E, Fernández-Garcés R (2015) Revision of the genera Parviturbo and Pseudorbis (Gastropoda, Skeneidae). Iberus 33(2):167–259. https://doi.org/10.5281/zenodo.4586816
Salas C (1996) Marine bivalves from off the Southern Iberian Peninsula collected by the Balgim and Fauna 1 expeditions. Haliotis 25:33–100
Salas C, Gofas S (1997) Brooding and non-brooding Dacrydium (Bivalvia, Mytilidae): a review of the Atlantic species. J Molluscan Stud 63(2):261–283. https://doi.org/10.1093/mollus/63.2.261
Sanders HL, Allen JA (1973) Studies on deep-sea Protobranchia (Bivalvia) Prologue and the Pristiglomidae. Bull Mus Comp Zool 145(6):237–262
Sanders HL, Allen JA (1977) Studies on deep-sea Protobranchia (Bivalvia). The family Tindariidae and the genus Pseudotindaria. Bull Mus Comp Zool 148(2):23–59
Sasaki T (2008) Micromolluscs in Japan: taxonomic composition, habitats, and future topics. Zoosymposia 1:147–232. https://doi.org/10.11646/zoosymposia.1.1.12
Segers W, Swinnen F, De Prins R (2009) Marine Molluscs of Madeira. Snoeck Publishers, Heule, p 612
Sokal RR, Rohlf FJ (1981) Biometry. W.H. Freeman & Co., New York, p 859
Strathmann RR (1985) Feeding and nonfeeding larval development and life-history evolution in marine invertebrates. Ann Rv Ecol Syst 16(1):339–361. https://doi.org/10.1146/annurev.es.16.110185.002011
Tucholke BE, Smoot NC (1990) Evidence for age and evolution of Corner Seamounts and Great Meteor Seamount Chain from multibeam bathymetry. J Geophys Res 95(B11):17555–17569. https://doi.org/10.1029/JB095iB11p17555
Valdés Á, Ortea J (1996) Review of the family Phyllidiidae in the Atlantic Ocean (Nudibranchia, Doridoidea). Am Malacol Bull 13(1–2):1–9
van Aartsen JJ, Gittenberger E, Goud J (1998) Pyramidellidae (Mollusca, Gastropoda, Heterobranchia) collected duning the Dutch CANCAP and MAURITANIA expeditions in the south-eastern part of the North Atlantic Ocean (part 1). Zool Verhand 321:1–57
van Aartsen JJ, Gittenberger E, Goud J (2000) Pyramidellidae (Mollusca, Gastropoda, Heterobranchia) collected during the Dutch CANCAP and MAURITANIA expeditions in the south-eastern part of the North Atlantic Ocean (part 2. Zool Meded 74:1–50
van der Linden J (1995) Philinidae dredged by the CANCAP expeditions (Gastropoda, Opisthobranchia). Basteria 59(1–3):65–83
van der Linden J (1998) The Metaxiinae dredged by the CANCAP expeditions, with the new species Metaxia carinapex and Metaxia hapax from the Cape Verde Islands (Gastropoda, Heteropoda: Triphoridae). Basteria 61(4/6):115–122
Verhecken A (2007) Revision of the Cancellariidae (Mollusca, Neogastropoda, Cancellarioidea) of the eastern Atlantic (40°N-40°S) and the Mediterranean. Zoosystema 29(2):281–364
Vermeij GJ (1989) Geographical restriction as a guide to the causes of extinction: the case of the cold northern oceans during the Neogene. Paleobiology 15(4):335–356
Warén A (1989a) New and little known Mollusca from Iceland. Sarsia 74(1):1–28. https://doi.org/10.1080/00364827.1989.10413419
Warén A (1989b) Taxonomic comments on some protobranch bivalves from the Northeastern Atlantic. Sarsia 74(4):223–259. https://doi.org/10.1080/00364827.1989.10413432
Warén A (1991) New and little known Mollusca from Iceland and Scandinavia. Sarsia 76(1–2):53–124. https://doi.org/10.1080/00364827.1991.10413466
Warén A (1992) New and little known “Skeneimorph” gastropods from the Mediterranean Sea and the adjacent Atlantic Ocean. Boll Malacol 27(10–12):149–248
Warén A (1993) New and little known Mollusca from Iceland and Scandinavia. Part 2. Sarsia 78(3–4):159–201. https://doi.org/10.1080/00364827.1993.10413534
Warén A (1996) New and little known Mollusca from Iceland and Scandinavia. Part 3. Sarsia 81(3):197–245. https://doi.org/10.1080/00364827.1996.10413622
Warén A (1996b) Ecology and systematics of the north European species of Rissoa and Pusillina (Prosobranchia: Rissoidae). J Mar Biol Assoc UK 76(4):1013–1059. https://doi.org/10.1017/S0025315400040947
Warén A, Bouchet P (1991) Mollusca Gastropoda: systematic position and revision of Haloceras Dall, 1889 (Caenogastropoda, Haloceratidae fam. nov.). In: Crosnier A et al (eds) Résultats des Campagnes MUSORSTOM 7. Mém Mus Nat Hist Nat, sér A, Zool 150: 111–161
Warén A, Gofas S (1996) Kaiparapelta askewi Mc Lean & Harasewych, 1995 (Gastropoda: Pseudococculinidae): a spongivorous cocculiniform limpet and a case of remarkable convergence in radular morphology. Haliotis 25:107–116
Warén A, Gofas S (1997) A new species of Monoplacophora, redescription of the genera Veleropilina and Rokopella, and new information on three species of the class. Zool Scr 25(3):215–232. https://doi.org/10.1111/j.1463-6409.1996.tb00163.x
Watling L, Guinotte J, Clark MR, Smith CR (2013) A proposed biogeography of the deep ocean floor. Prog Oceanog 111:91–112. https://doi.org/10.1016/j.pocean.2012.11.003
Watling L (2019) Macrofauna. In: Cochran JK, Bokuniewicz HJ, Yager PL (eds) Encyclopedia of Ocean Sciences. Elsevier/Academic Press, London, pp 728–734. https://doi.org/10.1016/B978-0-12-409548-9.11069-3
Williams ST, Kano Y, Warén A, Herbert DG (2020) Marrying molecules and morphology: first steps towards a reevaluation of solariellid genera (Gastropoda: Trochoidea) in the light of molecular phylogenetic studies. J Molluscan Stud 86(1):1–26. https://doi.org/10.1093/mollus/eyz038
Acknowledgements
We thank the captains, crews, and scientific staff for their assistance during the research cruises. We particularly acknowledge staff involved in the Seamount 2 (R/V Le Suroit) and M151 (R/V Meteor) cruises. Kai Horst George and Achim Wehrmann provided access to the POS397 (R/V Poseidon) samples. We are grateful to Mauricio R. Fernandes (UFRJ, Rio de Janeiro, Brazil) for a very thorough review of a previous draft of this work.
Funding
Funding for open access charge was provided by Universidad de Málaga/CBUA. Seamount 2 expedition was funded by Institut National des Sciences de l’Univers (INSU) of the French CNRS, with additional funding from Muséum National d’Histoire Naturelle for travel of participants. German cruises were funded by the Deutsche Forschungsgemeinschaft (DFG).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no competing interests.
Ethical approval
No animal testing was performed during this study.
Sampling and field studies
All necessary permits for sampling and observational field studies have been obtained from the competent authorities for the research expeditions involved in this study. The study is compliant with CBD and Nagoya protocols.
Data availability
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Author contributions
All authors contributed to the study conception and design and to drafting the manuscript. Serge Gofas was the expedition leader on Seamount 2. All authors participated in the specimen sorting and identification. The PRIMER and r-Macoqui analyses were performed by José Antonio Caballero Herrera. All authors commented on the drafts and approved the final manuscript.
Additional information
Communicated by M. Kaufmann
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Herrera, J.A.C., Hoffman, L., Freiwald, A. et al. The dispersal capacity of Mollusca—a test on the South Azorean Seamount Chain. Mar. Biodivers. 53, 59 (2023). https://doi.org/10.1007/s12526-023-01366-9
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12526-023-01366-9