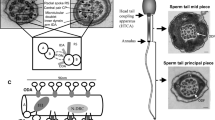
Abstract
Sperm motility is driven by motile cytoskeletal elements in the tail, called axonemes. The structure of axonemes consists of 9 + 2 microtubules, molecular motors (dyneins), and their regulatory structures. Axonemes are well conserved in motile cilia and flagella through eukaryotic evolution. Deficiency in the axonemal structure causes defects in sperm motility, and often leads to male infertility. It has been known since the 1970s that, in some cases, male infertility is linked with other symptoms or diseases such as Kartagener syndrome. Given that these links are mostly caused by deficiencies in the common components of cilia and flagella, they are called “immotile cilia syndrome” or “primary ciliary dyskinesia,” or more recently, “ciliopathy,” which includes deficiencies in primary and sensory cilia. Here, we review the structure of the sperm flagellum and epithelial cilia in the human body, and discuss how male fertility is linked to ciliopathy.




Similar content being viewed by others
Abbreviations
- AK:
-
Adenylate kinase
- ARMC:
-
Armadillo repeat containing
- BB:
-
Basal body
- BBS:
-
Bardet–Biedl syndrome
- CEP:
-
Centrosomal protein
- CCDC:
-
Coiled-coil domain-containing
- CP:
-
Central pair apparatus
- DC:
-
Docking complex
- DNAAF:
-
Dynein axonemal assembly factor
- DNAH:
-
Dynein, axonemal, heavy chain
- DNAI:
-
Dynein, axonemal, intermediate chain
- DNALI:
-
Dynein, axonemal, light intermediate chain
- DYNC2:
-
Dynein, cytoplasmic 2
- DYX1C1:
-
Dyslexia susceptibility 1 candidate 1
- FAP:
-
Flagella-associated protein
- FBB:
-
Flagellar basal body
- FoxJ1:
-
Forkhead box J1
- HC:
-
Heavy chain
- HEATR:
-
HEAT-repeat containing
- IC:
-
Intermediate chain
- IAD:
-
Inner arm dynein
- IDA:
-
Inner dynein arm
- IFT:
-
Intraflagellar transport
- IMT:
-
Intramanchette transport
- Iqcg:
-
IQ motif containing G
- LC:
-
Light chain
- LRRC:
-
Leucine-rich repeat containing
- MFN:
-
Mitofusin
- MIA:
-
Modifier of inner arms
- MKS:
-
McKusick–Kaufman syndrome
- MNS1:
-
Meiosis-specific nuclear structural 1
- N-DRC:
-
Nexin-dynein regulatory complex
- NPHP:
-
Nephronophthisis
- OAD:
-
Outer arm dynein
- ODA:
-
Outer dynein arm
- ODF:
-
Outer dense fiber
- OFD:
-
Oral-facial-digital syndrome
- PACRG:
-
Parkin co-regulated gene
- PCD:
-
Primary ciliary dyskinesia
- Pcdp1:
-
Primary ciliary dyskinesia protein 1
- PCP:
-
Planar cell polarity
- PF:
-
Paralyzed flagella
- PIH:
-
Protein interacting with HSP90
- PKD:
-
Polycystic kidney disease
- RFX:
-
Regulatory factor X
- RPGR:
-
Retinitis pigmentosa GTPase regulator
- RS:
-
Radial spoke
- SPAG:
-
Sperm-associated antigen
- Spef2:
-
Sperm flagellar protein 2
- TXNDC:
-
Thioredoxin domain containing
- TZ:
-
Transition zone
- VDAC3:
-
Voltage-dependent anion channel 3
- XLRP:
-
X-linked retinitis pigmentosa
- ZMYND:
-
Zinc-finger, MYND-type containing protein
References
Camner P, Mossberg B, Afzelius BA. Evidence of congenitally nonfunctioning cilia in the tracheobronchial tract in two subjects. Am Rev Respir Dis. 1975;112:807–9.
Afzelius BA. A human syndrome caused by immotile cilia. Science. 1976;193:317–9.
Afzelius BA. “Immotile-cilia” syndrome and ciliary abnormalities induced by infection and injury. Am Rev Respir Dis. 1981;124:107–9.
Afzelius BA. Situs inversus and ciliary abnormalities. What is the connection? Int J Dev Biol. 1995;39:839–44.
Afzelius BA. Cilia-related diseases. J Pathol. 2004;204:470–7.
Fawcett DW. The mammalian spermatozoon. Dev Biol. 1975;44:394–436.
Inaba K. Molecular architecture of the sperm flagella: molecules for motility and signaling. Zoolog Sci. 2003;20:1043–56.
Inaba K. Sperm flagella: comparative and phylogenetic perspectives of protein components. Mol Hum Reprod. 2011;17:524–38.
Darszon A, Nishigaki T, Beltran C, Treviño CL. Calcium channels in the development, maturation, and function of spermatozoa. Physiol Rev. 2011;91:1305–55.
Gibbons IR. Cilia and flagella of eukaryotes. J Cell Biol. 1981;91:107s–24s.
Mohri H, Inaba K, Ishijima S, Baba SA. Tubulin–dynein system in flagellar and ciliary movement. Proc Jpn Acad Ser B Phys Biol Sci. 2012;88:397–415.
Summers KE, Gibbons IR. Adenosine triphosphate-induced sliding of tubules in trypsin-treated flagella of sea-urchin sperm. Proc Natl Acad Sci USA. 1971;68:3092–6.
Kamiya R. Functional diversity of axonemal dyneins as studied in Chlamydomonas mutants. Int Rev Cytol. 2002;219:115–55.
Smith EF, Sale WS. Regulation of dynein-driven microtubule sliding by the radial spokes in flagella. Science. 1992;257:1557–9.
Smith EF, Yang P. The radial spokes and central apparatus: mechano-chemical transducers that regulate flagellar motility. Cell Motil Cytoskeleton. 2004;57:8–17.
Kamiya R. Mutations at twelve independent loci result in absence of outer dynein arms in Chlamydomonas reinhardtii. J Cell Biol. 1988;107:2253–8.
Huang B, Piperno G, Luck DJ. Paralyzed flagella mutants of Chlamydomonas reinhardtii. Defective for axonemal doublet microtubule arms. J Biol Chem. 1979;254:3091–9.
Gibbons BH, Gibbons IR. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with triton X-100. J Cell Biol. 1972;54:75–97.
Gibbons BH, Gibbons IR. The effect of partial extraction of dynein arms on the movement of reactivated sea-urchin sperm. J Cell Sci. 1973;13:337–57.
Shingyoji C, Murakami A, Takahashi K. Local reactivation of Triton-extracted flagella by iontophoretic application of ATP. Nature. 1977;265:269–70.
Brokaw CJ. Direct measurements of sliding between outer doublet microtubules in swimming sperm flagella. Science. 1989;243:1593–6.
Okuno M, Brokaw CJ. Inhibition of movement of trition-demembranated sea-urchin sperm flagella by Mg2+, ATP4−, ADP and P1. J Cell Sci. 1979;38:105–23.
Tang WJ, Bell CW, Sale WS, Gibbons IR. Structure of the dynein-1 outer arm in sea urchin sperm flagella. I. Analysis by separation of subunits. J Biol Chem. 1982;257:508–15.
Ishijima S. Mechanical constraint converts planar waves into helices on tunicate and sea urchin sperm flagella. Cell Struct Funct. 2012;37:13–9.
King SM. The dynein microtubule motor. Biochim Biophys Acta. 2000;1496:60–75.
Gibbons IR, Gibbons BH, Mocz G, Asai DJ. Multiple nucleotide-binding sites in the sequence of dynein beta heavy chain. Nature. 1991;352:640–3.
Ogawa K. Four ATP-binding sites in the midregion of the β heavy chain of dynein. Nature. 1991;352:643–5.
Inaba K. Molecular basis of sperm flagellar axonemes: structural and evolutionary aspects. Ann N Y Acad Sci. 2007;1101:506–26.
Inaba K, Mizuno K, Shiba K. Structure, function, and phylogenetic consideration of calaxin. In: Sawada H, Inoue N, Iwano M, editors. Sexual reproduction in animals and plants. Springer: Berlin; 2014. p. 49–51.
Ogawa K, Takai H, Ogiwara A, Yokota E, Shimizu T, Inaba K, et al. Is outer arm dynein intermediate chain 1 multifunctional? Mol Biol Cell. 1996;7:1895–907.
Padma P, Hozumi A, Ogawa K, Inaba K. Molecular cloning and characterization of a thioredoxin/nucleoside diphosphate kinase related dynein intermediate chain from the ascidian, Ciona intestinalis. Gene. 2001;275:177–83.
Sadek CM, Damdimopoulos AE, Pelto-Huikko M, Gustafsson JA, Spyrou G, Miranda-Vizuete A. Sptrx-2, a fusion protein composed of one thioredoxin and three tandemly repeated NDP-kinase domains is expressed in human testis germ cells. Genes Cells. 2001;6:1077–90.
Inaba K, Morisawa S, Morisawa M. Proteasomes regulate the motility of salmonid fish sperm through modulation of cAMP-dependent phosphorylation of an outer arm dynein light chain. J Cell Sci. 1998;111:1105–15.
Inaba K, Kagami O, Ogawa K. Tctex2-related outer arm dynein light chain is phosphorylated at activation of sperm motility. Biochem Biophys Res Commun. 1999;256:177–83.
Mizuno K, Padma P, Konno A, Satouh Y, Ogawa K, Inaba K. A novel neuronal calcium sensor family protein, calaxin, is a potential Ca2+-dependent regulator for the outer arm dynein of metazoan cilia and flagella. Biol Cell. 2009;101:91–103.
Mizuno K, Shiba K, Okai M, Takahashi Y, Shitaka Y, Oiwa K, et al. Calaxin drives sperm chemotaxis by Ca2+-mediated direct modulation of a dynein motor. Proc Natl Acad Sci USA. 2012;109:20497–502.
Inaba K. Calcium sensors of ciliary outer arm dynein: functions and phylogenetic considerations for eukaryotic evolution. Cilia. 2015;4:6.
Takada S, Wilkerson CG, Wakabayashi K, Kamiya R, Witman GB. The outer dynein arm-docking complex: composition and characterization of a subunit (oda1) necessary for outer arm assembly. Mol Biol Cell. 2002;13:1015–29.
Owa M, Furuta A, Usukura J, Arisaka F, King SM, Witman GB, Kamiya R, Wakabayashi K. Cooperative binding of the outer arm-docking complex underlies the regular arrangement of outer arm dynein in the axoneme. Proc Natl Acad Sci USA. 2012;111:9461–6.
Hozumi A, Satouh Y, Makino Y, Toda T, Ide H, Ogawa K, et al. Molecular characterization of Ciona sperm outer arm dynein reveals multiple components related to outer arm docking complex protein 2. Cell Motil Cytoskeleton. 2006;63:591–603.
Kamiya R, Yagi T. Functional diversity of axonemal dyneins as assessed by in vitro and in vivo motility assays of Chlamydomonas mutants. Zoolog Sci. 2014;31:633–44.
Wada S, Okuno M, Mohri H. Inner arm dynein ATPase fraction of sea urchin sperm flagella causes active sliding of axonemal outer doublet microtubule. Biochem Biophys Res Commun. 1991;175:173–8.
Yokota E, Mabuchi I. Isolation and characterization of a novel dynein that contains C and A heavy chains from sea urchin sperm flagellar axonemes. J Cell Sci. 1994;107:345–51.
Hozumi A, Padma P, Toda T, Ide H, Inaba K. Molecular characterization of axonemal proteins and signaling molecules responsible for chemoattractant-induced sperm activation in Ciona intestinalis. Cell Motil Cytoskeleton. 2008;65:249–67.
Huang B, Ramanis Z, Luck DJ. Suppressor mutations in Chlamydomonas reveal a regulatory mechanism for flagellar function. Cell. 1982;28:115–24.
Gardner LC, O’Toole E, Perrone CA, Giddings T, Porter ME. Components of a “dynein regulatory complex” are located at the junction between the radial spokes and the dynein arms in Chlamydomonas flagella. J Cell Biol. 1994;127:1311–25.
Downing KH, Sui H. Structural insights into microtubule doublet interactions in axonemes. Curr Opin Struct Biol. 2007;17:253–9.
Heuser T, Raytchev M, Krell J, Porter ME, Nicastro D. The dynein regulatory complex is the nexin link and a major regulatory node in cilia and flagella. J Cell Biol. 2009;187:921–33.
Bui KH, Sakakibara H, Movassagh T, Oiwa K, Ishikawa T. Asymmetry of inner dynein arms and inter-doublet links in Chlamydomonas flagella. J Cell Biol. 2009;186:437–46.
Mitchell DR, Sale WS. Characterization of a Chlamydomonas insertional mutant that disrupts flagellar central pair microtubule-associated structures. J Cell Biol. 1999;144:293–304.
Nicastro D, Fu X, Heuser T, Tso A, Porter ME, Linck RW. Cryo-electron tomography reveals conserved features of doublet microtubules in flagella. Proc Natl Acad Sci USA. 2011;108:E845–53.
Hoops HJ, Witman GB. Outer doublet heterogeneity reveals structural polarity related to beat direction in Chlamydomonas flagella. J Cell Biol. 1983;97:902–8.
Mukai C, Okuno M. Glycolysis plays a major role for adenosine triphosphate supplementation in mouse sperm flagellar movement. Biol Reprod. 2004;71:540–7.
Miki K, Qu W, Goulding EH, Willis WD, Bunch DO, Strader LF, et al. Glyceraldehyde 3-phosphate dehydrogenase-S, a sperm-specific glycolytic enzyme, is required for sperm motility and male fertility. Proc Natl Acad Sci USA. 2004;101:16501–6.
Anderson RG. The three-dimensional structure of the basal body from the rhesus monkey oviduct. J Cell Biol. 1972;54:246–65.
Bornens M. Organelle positioning and cell polarity. Nat Rev Mol Cell Biol. 2008;9:874–86.
Boisvieux-Ulrich E, Sandoz D. Determination of ciliary polarity precedes differentiation in the epithelial cells of quail oviduct. Biol Cell. 1991;72:3–14.
Nachury MV, Loktev AV, Zhang Q, Westlake CJ, Peränen J, Merdes A, et al. A core complex of BBS proteins cooperates with the GTPase Rab8 to promote ciliary membrane biogenesis. Cell. 2007;129:1201–13.
Donaldson JG, Jackson CL. ARF family G proteins and their regulators: roles in membrane transport, development and disease. Nat Rev Mol Cell Biol. 2011;12:362–75.
Kozminski KG, Beech PL, Rosenbaum JL. The Chlamydomonas kinesin-like protein FLA10 is involved in motility associated with the flagellar membrane. J Cell Biol. 1995;131:1517–27.
Rosenbaum JL, Witman GB. Intraflagellar transport. Nat Rev Mol Cell Biol. 2002;3:813–25.
Sarpal R, Todi SV, Sivan-Loukianova E, Shirolikar S, Subramanian N, Raff EC, et al. Drosophila KAP interacts with the kinesin II motor subunit KLP64D to assemble chordotonal sensory cilia, but not sperm tails. Curr Biol. 2003;13:1687–96.
Han YG, Kwok BH, Kernan MJ. Intraflagellar transport is required in Drosophila to differentiate sensory cilia but not sperm. Curr Biol. 2003;13:1679–86.
Manandhar G, Sutovsky P, Joshi HC, Stearns T, Schatten G. Centrosome reduction during mouse spermiogenesis. Dev Biol. 1998;203:424–34.
Manandhar G, Simerly C, Schatten G. Highly degenerated distal centrioles in rhesus and human spermatozoa. Hum Reprod. 2000;15:256–63.
Fawcett DW, Phillips DM. The fine structure and development of the neck region of the mammalian spermatozoon. Anat Rec. 1969;165:153–64.
Konno A, Shiba K, Cai C, Inaba K. Branchial cilia and sperm flagella recruit distinct axonemal components. PLoS One. 2015;10:e0126005.
Li X, Xu L, Li J, Li B, Bai X, Strauss JF 3rd, et al. Otitis media in sperm-associated antigen 6 (Spag6)-deficient mice. PLoS One. 2014;9:e112879.
Dabdoub A, Kelley MW. Planar cell polarity and a potential role for a Wnt morphogen gradient in stereociliary bundle orientation in the mammalian inner ear. J Neurobiol. 2005;64:446–57.
Sobkowicz HM, Slapnick SM, August BK. The kinocilium of auditory hair cells and evidence for its morphogenetic role during the regeneration of stereocilia and cuticular plates. J Neurocytol. 1995;24:633–53.
Kikuchi T, Takasaka T, Tonosaki A, Watanabe H. Fine structure of guinea pig vestibular kinocilium. Acta Otolaryngol. 1989;108:26–30.
Sobrinho-Simões M, Johannessen JV. Scanning electron microscopy of the normal human thyroid. J Submicrosc Cytol. 1981;13:209–22.
Martin A, Hedinger C, Häberlin-Jakob M, Walt H. Structure and motility of primary cilia in the follicular epithelium of the human thyroid. Virchows Arch B Cell Pathol Incl Mol Pathol. 1988;55:159–66.
Robaire B, Hinton BT, Orgebin-Crist MC. The epididymis. In: Neill JD, editor. Knobil and Neill’s physiology of reproduction. 3rd ed. Elsevier: Amsterdam; 2006. p. 1071–148.
Kormanko M, Reijonen K. Microvascular structure of human epididymis. Am J Anat. 1976;145:23–32.
Paniagua R, Regadera J, Nistal M, Abaurrea MA. Histological, histochemical and ultrastructural variations along the length of the human vas deferens before and after puberty. Acta Anat. 1982;111:190–203.
Hando T, Okada DM, Zamboni L. Atypical cilia in human endometrium. J Cell Biol. 1968;39:475–81.
Denholm RB, More IA. Atypical cilia of the human endometrial epithelium. J Anat. 1980;131:309–15.
Eliyahu S, Shalev E. A fertile woman with Kartagener’s syndrome and three consecutive pregnancies. Hum Reprod. 1996;11:683.
Singla V, Reiter JF. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science. 2006;313:629–33.
Nonaka S, Tanaka Y, Okada Y, Takeda S, Harada A, Kanai Y, et al. Randomization of left–right asymmetry due to loss of nodal cilia generating leftward flow of extraembryonic fluid in mice lacking KIF3B motor protein. Cell. 1998;95:829–37.
Takeda S, Yonekawa Y, Tanaka Y, Okada Y, Nonaka S, Hirokawa N. Left–right asymmetry and kinesin superfamily protein KIF3A: new insights in determination of laterality and mesoderm induction by kif3A−/− mice analysis. J Cell Biol. 1999;145:825–36.
Marshall WF, Nonaka S. Cilia: tuning into the cell’s antenna. Curr Biol. 2006;16:R604–14.
Hirokawa N, Tanaka Y, Okada Y, Takeda S. Nodal flow and the generation of left–right asymmetry. Cell. 2006;125:33–45.
Shinohara K, Kawasumi A, Takamatsu A, Yoshiba S, Botilde Y, Motoyama N, et al. Two rotating cilia in the node cavity are sufficient to break left–right symmetry in the mouse embryo. Nat Commun. 2012;3:622.
McGrath J, Somlo S, Makova S, Tian X, Brueckner M. Two populations of node monocilia initiate left–right asymmetry in the mouse. Cell. 2003;114:61–73.
Babu D, Roy S. Left–right asymmetry: cilia stir up new surprises in the node. Open Biol. 2013;3:130052.
Bangs FK, Schrode N, Hadjantonakis AK, Anderson KV. Lineage specificity of primary cilia in the mouse embryo. Nat Cell Biol. 2015;17:113–22.
Slough J, Cooney L, Brueckner M. Monocilia in the embryonic mouse heart suggest a direct role for cilia in cardiac morphogenesis. Dev Dyn. 2008;237:2304–14.
Thomas J, Morlé L, Soulavie F, Laurençon A, Sagnol S, Durand B. Transcriptional control of genes involved in ciliogenesis: a first step in making cilia. Biol Cell. 2010;102:499–513.
Reiter JF, Blacque OE, Leroux MR. The base of the cilium: roles for transition fibres and the transition zone in ciliary formation, maintenance and compartmentalization. EMBO Rep. 2012;13:608–18.
Tobin JL, Beales PL. The nonmotile ciliopathies. Genet Med. 2009;11:386–402.
Zaghloul NA, Katsanis N. Mechanistic insights into Bardet–Biedl syndrome, a model ciliopathy. J Clin Invest. 2009;119:428–37.
Norris DP, Grimes DT. Mouse models of ciliopathies: the state of the art. Dis Model Mech. 2012;5:299–312.
Mykytyn K, Mullins RF, Andrews M, Chiang AP, Swiderski RE, Yang B, et al. Bardet–Biedl syndrome type 4 (BBS4)-null mice implicate Bbs4 in flagella formation but not global cilia assembly. Proc Natl Acad Sci USA. 2004;101:8664–9.
Sun Z, Amsterdam A, Pazour GJ, Cole DG, Miller MS, Hopkins N. A genetic screen in zebrafish identifies cilia genes as a principal cause of cystic kidney. Development. 2004;131:4085–93.
Gorivodsky M, Mukhopadhyay M, Wilsch-Braeuninger M, Phillips M, Teufel A, Kim C, et al. Intraflagellar transport protein 172 is essential for primary cilia formation and plays a vital role in patterning the mammalian brain. Dev Biol. 2009;325:24–32.
Murcia NS, Richards WG, Yoder BK, Mucenski ML, Dunlap JR, Woychik RP. The Oak Ridge Polycystic Kidney (orpk) disease gene is required for left–right axis determination. Development. 2000;127:2347–55.
Pazour GJ, Dickert BL, Vucica Y, Seeley ES, Rosenbaum JL, Witman GB, et al. Chlamydomonas IFT88 and its mouse homologue, polycystic kidney disease gene tg737, are required for assembly of cilia and flagella. J Cell Biol. 2000;151:709–18.
Iomini C, Babaev-Khaimov V, Sassaroli M, Piperno G. Protein particles in Chlamydomonas flagella undergo a transport cycle consisting of four phases. J Cell Biol. 2001;153:13–24.
Iomini C, Li L, Esparza JM, Dutcher SK. Retrograde intraflagellar transport mutants identify complex A proteins with multiple genetic interactions in Chlamydomonas reinhardtii. Genetics. 2009;183:885–96.
Liem KF Jr, Ashe A, He M, Satir P, Moran J, Beier D, et al. The IFT-A complex regulates Shh signaling through cilia structure and membrane protein trafficking. J Cell Biol. 2012;197:789–800.
Mill P, Lockhart PJ, Fitzpatrick E, Mountford HS, Hall EA, Reijns MA, et al. Human and mouse mutations in WDR35 cause short-rib polydactyly syndromes due to abnormal ciliogenesis. Am J Hum Genet. 2011;88:508–15.
Perrault I, Saunier S, Hanein S, Filhol E, Bizet AA, Collins F, et al. Mainzer–Saldino syndrome is a ciliopathy caused by IFT140 mutations. Am J Hum Genet. 2012;90:864–70.
Byers B, Goetsch L. A highly ordered ring of membrane-associated filaments in budding yeast. J Cell Biol. 1976;69:717–21.
Bezanilla M, Gladfelter AS, Kovar DR, Lee WL. Cytoskeletal dynamics: a view from the membrane. J Cell Biol. 2015;209:329–37.
Hu Q, Milenkovic L, Jin H, Scott MP, Nachury MV, Spiliotis ET, et al. A septin diffusion barrier at the base of the primary cilium maintains ciliary membrane protein distribution. Science. 2010;329:436–9.
Ihara M, Kinoshita A, Yamada S, Tanaka H, Tanigaki A, Kitano A, et al. Cortical organization by the septin cytoskeleton is essential for structural and mechanical integrity of mammalian spermatozoa. Dev Cell. 2005;8:343–52.
Kissel H, Georgescu MM, Larisch S, Manova K, Hunnicutt GR, Steller H. The Sept4 septin locus is required for sperm terminal differentiation in mice. Dev Cell. 2005;8:353–64.
Kuo YC, Lin YH, Chen HI, Wang YY, Chiou YW, Lin HH, et al. SEPT12 mutations cause male infertility with defective sperm annulus. Hum Mutat. 2012;33:710–9.
Craige B, Tsao CC, Diener DR, Hou Y, Lechtreck KF, Rosenbaum JL, Witman GB. CEP290 tethers flagellar transition zone microtubules to the membrane and regulates flagellar protein content. J Cell Biol. 2010;190:927–40.
Baala L, Audollent S, Martinovic J, Ozilou C, Babron MC, Sivanandamoorthy S, et al. Pleiotropic effects of CEP290 (NPHP6) mutations extend to Meckel syndrome. Am J Hum Genet. 2007;81:170–9.
Ishikawa H, Kubo A, Tsukita S, Tsukita S. Odf2-deficient mother centrioles lack distal/subdistal appendages and the ability to generate primary cilia. Nat Cell Biol. 2005;7:517–24.
Delgehyr N, Sillibourne J, Bornens M. Microtubule nucleation and anchoring at the centrosome are independent processes linked by ninein function. J Cell Sci. 2005;118:1565–75.
Singla V, Romaguera-Ros M, Garcia-Verdugo JM, Reiter JF. Ofd1, a human disease gene, regulates the length and distal structure of centrioles. Dev Cell. 2010;18:410–24.
Sillibourne JE, Specht CG, Izeddin I, Hurbain I, Tran P, Triller A, et al. Assessing the localization of centrosomal proteins by PALM/STORM nanoscopy. Cytoskeleton. 2011;68:619–27.
Kunimoto K, Yamazaki Y, Nishida E, Shinohara K, Ishikawa H, Hasegawa T, et al. Coordinated ciliary beating requires Odf2-mediated polarization of basal bodies via basal feet. Cell. 2012;148:189–200.
Salmon NA, Reijo Pera RA, Xu EY. A gene trap knockout of the abundant sperm tail protein, outer dense fiber 2, results in preimplantation lethality. Genesis. 2006;44:515–22.
Shao X, Tarnasky HA, Schalles U, Oko R, van der Hoorn FA. Interactional cloning of the 84-kDa major outer dense fiber protein Odf84. Leucine zippers mediate associations of Odf84 and Odf27. J Biol Chem. 1997;272:6105–13.
Tarnasky H, Cheng M, Ou Y, Thundathil JC, Oko R, van der Hoorn FA. Gene trap mutation of murine Outer dense fiber protein-2 gene can result in sperm tail abnormalities in mice with high percentage chimaerism. BMC Dev Biol. 2010;10:67.
Lechtreck KF, Melkonian M. Striated microtubule-associated fibers: identification of assemblin, a novel 34-kD protein that forms paracrystals of 2-nm filaments in vitro. J Cell Biol. 1991;115:705–16.
Yang J, Liu X, Yue G, Adamian M, Bulgakov O, Li T. Rootletin, a novel coiled-coil protein, is a structural component of the ciliary rootlet. J Cell Biol. 2002;159:431–40.
Gilliam JC, Chang JT, Sandoval IM, Zhang Y, Li T, Pittler SJ, Chiu W, et al. Three-dimensional architecture of the rod sensory cilium and its disruption in retinal neurodegeneration. Cell. 2012;151:1029–41.
Roosing S, Rohrschneider K, Beryozkin A, Sharon D, Weisschuh N, Staller J, et al. Mutations in RAB28, encoding a farnesylated small GTPase, are associated with autosomal-recessive cone-rod dystrophy. Am J Hum Genet. 2013;93:110–7.
Omran H, Kobayashi D, Olbrich H, Tsukahara T, Loges NT, Hagiwara H, et al. Ktu/PF13 is required for cytoplasmic pre-assembly of axonemal dyneins. Nature. 2008;456:611–6.
Duquesnoy P, Escudier E, Vincensini L, Freshour J, Bridoux AM, Coste A, et al. Loss-of-function mutations in the human ortholog of Chlamydomonas reinhardtii ODA7 disrupt dynein arm assembly and cause primary ciliary dyskinesia. Am J Hum Genet. 2009;85:890–6.
Loges NT, Olbrich H, Becker-Heck A, Häffner K, Heer A, Reinhard C, et al. Deletions and point mutations of LRRC50 cause primary ciliary dyskinesia due to dynein arm defects. Am J Hum Genet. 2009;85:883–9.
Mitchison HM, Schmidts M, Loges NT, Freshour J, Dritsoula A, Hirst RA, et al. Mutations in axonemal dynein assembly factor DNAAF3 cause primary ciliary dyskinesia. Nat Genet. 2012;44:381–9.
Tarkar A, Loges NT, Slagle CE, Francis R, Dougherty GW, Tamayo JV, et al. DYX1C1 is required for axonemal dynein assembly and ciliary motility. Nat Genet. 2013;45:995–1003.
Horani A, Druley TE, Zariwala MA, Patel AC, Levinson BT, Van Arendonk LG, et al. Whole-exome capture and sequencing identifies HEATR2 mutation as a cause of primary ciliary dyskinesia. Am J Hum Genet. 2012;91:685–93.
Kott E, Legendre M, Copin B, Papon JF, Dastot-Le Moal F, Montantin G, et al. Loss-of-function mutations in RSPH1 cause primary ciliary dyskinesia with central-complex and radial-spoke defects. Am J Hum Genet. 2013;93:561–70.
Moore A, Escudier E, Roger G, Tamalet A, Pelosse B, Marlin S, et al. RPGR is mutated in patients with a complex X linked phenotype combining primary ciliary dyskinesia and retinitis pigmentosa. J Med Genet. 2006;43:326–33.
Zariwala MA, Gee HY, Kurkowiak M, Al-Mutairi DA, Leigh MW, Hurd TW, et al. ZMYND10 is mutated in primary ciliary dyskinesia and interacts with LRRC6. Am J Hum Genet. 2013;93:336–45.
Knowles MR, Ostrowski LE, Loges NT, Hurd T, Leigh MW, Huang L, et al. Mutations in SPAG1 cause primary ciliary dyskinesia associated with defective outer and inner dynein arms. Am J Hum Genet. 2013;93:711–20.
Yamamoto R, Hirono M, Kamiya R. Discrete PIH proteins function in the cytoplasmic preassembly of different subsets of axonemal dyneins. J Cell Biol. 2010;190:65–71.
Yamamoto R, Song K, Yanagisawa HA, Fox L, Yagi T, Wirschell M, et al. The MIA complex is a conserved and novel dynein regulator essential for normal ciliary motility. J Cell Biol. 2013;201:263–78.
Olbrich H, Schmidts M, Werner C, Onoufriadis A, Loges NT, Raidt J, et al. Recessive HYDIN mutations cause primary ciliary dyskinesia without randomization of left–right body asymmetry. Am J Hum Genet. 2012;91:672–84.
Castleman VH, Romio L, Chodhari R, Hirst RA, de Castro SC, Parker KA, et al. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. Am J Hum Genet. 2009;84:197–209.
Papon JF, Coste A, Roudot-Thoraval F, Boucherat M, Roger G, Tamalet A, et al. A 20-year experience of electron microscopy in the diagnosis of primary ciliary dyskinesia. Eur Respir J. 2010;35:1057–63.
Shoemark A, Dixon M, Corrin B, Dewar A. Twenty-year review of quantitative transmission electron microscopy for the diagnosis of primary ciliary dyskinesia. J Clin Pathol. 2012;65:267–71.
Wolf JP, Feneux D, Escalier D, Rodrigues D, Frydman R, Jouannet P. Pregnancy after subzonal insemination with spermatozoa lacking outer dynein arms. J Reprod Fertil. 1993;97:487–92.
Rompolas P, Patel-King RS, King SM. An outer arm dynein conformational switch is required for metachronal synchrony of motile cilia in planaria. Mol Biol Cell. 2010;21:3669–79.
Neesen J, Kirschner R, Ochs M, Schmiedl A, Habermann B, Mueller C, et al. Disruption of an inner arm dynein heavy chain gene results in asthenozoospermia and reduced ciliary beat frequency. Hum Mol Genet. 2001;10:1117–28.
Shoemark A, Ives A, Becker-Heck A, Burgoyne T, Dixon M, Bilton D, et al. Inner dynein arm defects in primary ciliary dyskinesia. J Genet Syndr Gene Ther. 2013;4:7.
Kobayashi Y, Watanabe M, Okada Y, Sawa H, Takai H, Nakanishi M, et al. Hydrocephalus, situs inversus, chronic sinusitis, and male infertility in DNA polymerase lambda-deficient mice: possible implication for the pathogenesis of immotile cilia syndrome. Mol Cell Biol. 2002;22:2769–76.
Zariwala M, O’Neal WK, Noone PG, Leigh MW, Knowles MR, Ostrowski LE. Investigation of the possible role of a novel gene, DPCD, in primary ciliary dyskinesia. Am J Respir Cell Mol Biol. 2004;30:428–34.
Fliegauf M, Olbrich H, Horvath J, Wildhaber JH, Xariwala MA, Kennedy M, et al. Mislocalization of DNAH5 and DNAH9 in respiratory cells from patients with primary ciliary dyskinesia. Am J Respir Crit Care Med. 2005;171:1343–9.
Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left–right asymmetry in inversus viscerum mice. Nature. 1997;389:963–6.
Bartoloni L, Blouin JL, Pan Y, Gehrig C, Maiti AK, Scamuffa N, et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proc Natl Acad Sci USA. 2002;99:10282–6.
Schwabe GC, Hoffmann K, Loges NT, Birker D, Rossier C, de Santi MM, et al. Primary ciliary dyskinesia associated with normal axoneme ultrastructure is caused by DNAH11 mutations. Hum Mutat. 2008;29:289–98.
Inaba K. Regulatory subunits of axonemal dynein. In: Hirose K, Amos LA, editors. Handbook of dynein. Singapore: Pan Stanford; 2011. p. 304–24.
Ushimaru Y, Konno A, Kaizu M, Ogawa K, Satoh N, Inaba K. Association of a 66 kDa homolog of Chlamydomonas DC2, a subunit of the outer arm docking complex, with outer arm dynein of sperm flagella in the ascidian Ciona intestinalis. Zoolog Sci. 2006;23:679–87.
Onoufriadis A, Paff T, Antony D, Shoemark A, Micha D, Kuyt B, et al. Splice-site mutations in the axonemal outer dynein arm docking complex gene CCDC114 cause primary ciliary dyskinesia. Am J Hum Genet. 2013;92:88–98.
Satouh Y, Inaba K. Proteomic characterization of sperm radial spokes identifies a novel spoke protein with an ubiquitin domain. FEBS Lett. 2009;583:2201–7.
Wirschell M, Pazour G, Yoda A, Hirono M, Kamiya R, Witman GB. Oda5p, a novel axonemal protein required for assembly of the outer dynein arm and an associated adenylate kinase. Mol Biol Cell. 2004;15:2729–41.
Dean AB, Mitchell DR. Chlamydomonas ODA10 is a conserved axonemal protein that plays a unique role in outer dynein arm assembly. Mol Biol Cell. 2013;24:3689–96.
Desai PB, Freshour JR, Mitchell DR. Chlamydomonas axonemal dynein assembly locus ODA8 encodes a conserved flagellar protein needed for cytoplasmic maturation of outer dynein arm complexes. Cytoskeleton. 2015;72:16–28.
Ahmed NT, Mitchell DR. ODA16p, a Chlamydomonas flagellar protein needed for dynein assembly. Mol Biol Cell. 2005;16:5004–12.
Ahmed NT, Gao C, Lucker BF, Cole DG, Mitchell DR. ODA16 aids axonemal outer row dynein assembly through an interaction with the intraflagellar transport machinery. J Cell Biol. 2008;183:313–22.
Hjeij R, Lindstrand A, Francis R, Zariwala MA, Liu X, Li Y, et al. ARMC4 mutations cause primary ciliary dyskinesia with randomization of left/right body asymmetry. Am J Hum Genet. 2013;93:357–67.
Panizzi JR, Becker-Heck A, Castleman VH, Al-Mutairi DA, Liu Y, Loges NT, et al. CCDC103 mutations cause primary ciliary dyskinesia by disrupting assembly of ciliary dynein arms. Nat Genet. 2012;44:714–9.
King SM, Patel-King RS. The oligomeric outer dynein arm assembly factor CCDC103 is tightly integrated within the ciliary axoneme and exhibits periodic binding to microtubules. J Biol Chem. 2015;290:7388–401.
Austin-Tse C, Halbritter J, Zariwala MA, Gilberti RM, Gee HY, Hellman N, et al. Zebrafish ciliopathy screen plus human mutational analysis identifies C21orf59 and CCDC65 defects as causing primary ciliary dyskinesia. Am J Hum Genet. 2013;93:672–86.
Sale WS. The axonemal axis and Ca2+-induced asymmetry of active microtubule sliding in sea urchin sperm tails. J Cell Biol. 1986;102:2042–52.
Nakano I, Kobayashi T, Yoshimura M, Shingyoji C. Central-pair-linked regulation of microtubule sliding by calcium in flagellar axonemes. J Cell Sci. 2003;116:1627–36.
Piperno G, Mead K, Shestak W. The inner dynein arms I2 interact with a “dynein regulatory complex” in Chlamydomonas flagella. J Cell Biol. 1992;118:1455–63.
Merveille AC, Davis EE, Becker-Heck A, Legendre M, Amirav I, Bataille G, et al. CCDC39 is required for assembly of inner dynein arms and the dynein regulatory complex and for normal ciliary motility in humans and dogs. Nat Genet. 2011;43:72–8.
Becker-Heck A, Zohn IE, Okabe N, Pollock A, Lenhart KB, Sullivan-Brown J, et al. The coiled-coil domain containing protein CCDC40 is essential for motile cilia function and left–right axis formation. Nat Genet. 2011;43:79–84.
Wirschell M, Olbrich H, Werner C, Tritschler D, Bower R, Sale WS, et al. The nexin-dynein regulatory complex subunit DRC1 is essential for motile cilia function in algae and humans. Nat Genet. 2013;45:262–8.
Budny B, Chen W, Omran H, Fliegauf M, Tzschach A, Wisniewska M, et al. A novel X-linked recessive mental retardation syndrome comprising macrocephaly and ciliary dysfunction is allelic to oral-facial-digital type I syndrome. Hum Genet. 2006;120:171–8.
Bennett WI, Gall AM, Southard JL, Sidman RL. Abnormal spermiogenesis in quaking, a myelin-deficient mutant mouse. Biol Reprod. 1971;5:30–58.
Lorenzetti D, Bishop CE, Justice MJ. Deletion of the Parkin coregulated gene causes male sterility in the quakingviable mouse mutant. Proc Natl Acad Sci USA. 2004;101:8402–7.
Dawe HR, Farr H, Portman N, Shaw MK, Gull K. The Parkin co-regulated gene product, PACRG, is an evolutionarily conserved axonemal protein that functions in outer-doublet microtubule morphogenesis. J Cell Sci. 2005;118:5421–30.
Ikeda K, Ikeda T, Morikawa K, Kamiya R. Axonemal localization of Chlamydomonas PACRG, a homologue of the human Parkin-coregulated gene product. Cell Motil Cytoskeleton. 2007;64:814–21.
Zhang Z, Kostetskii I, Tang W, Haig-Ladewig L, Sapiro R, Wei Z, et al. Deficiency of SPAG16L causes male infertility associated with impaired sperm motility. Biol Reprod. 2006;74:751–9.
Zhang Z, Shen X, Gude DR, Wilkinson BM, Justice MJ, Flickinger CJ, et al. MEIG1 is essential for spermiogenesis in mice. Proc Natl Acad Sci USA. 2009;106:17055–60.
Salzberg Y, Eldar T, Karminsky OD, Itach SB, Pietrokovski S, Don J. Meig1 deficiency causes a severe defect in mouse spermatogenesis. Dev Biol. 2010;338:158–67.
Teves ME, Jha KN, Song J, Nagarkatti-Gude DR, Herr JC, Foster JA, et al. Germ cell-specific disruption of the Meig1 gene causes impaired spermiogenesis in mice. Andrology. 2013;1:37–46.
Li W, Tang W, Teves ME, Zhang Z, Zhang L, Li H, et al. A MEIG1/PACRG complex in the manchette is essential for building the sperm flagella. Development. 2015;142:921–30.
Kierszenbaum AL. Spermatid manchette: plugging proteins to zero into the sperm tail. Mol Reprod Dev. 2001;59:347–9.
Pan Q, Zhu YJ, Gu BW, Cai X, Bai XT, Yun HY, et al. A new fusion gene NUP98-IQCG identified in an acute T-lymphoid/myeloid leukemia with a t(3;11)(q29q13;p15)del(3)(q29) translocation. Oncogene. 2008;27:3414–23.
Li RK, Tan JL, Chen LT, Feng JS, Liang WX, Guo XJ, et al. Iqcg is essential for sperm flagellum formation in mice. PLoS One. 2014;9:e98053.
Lee L, Campagna DR, Pinkus JL, Mulhern H, Wyatt TA, Sisson JH, et al. Primary ciliary dyskinesia in mice lacking the novel ciliary protein Pcdp1. Mol Cell Biol. 2008;28:949–57.
DiPetrillo CG, Smith EF. Pcdp1 is a central apparatus protein that binds Ca2+-calmodulin and regulates ciliary motility. J Cell Biol. 2010;189:601–12.
Sironen A, Hansen J, Thomsen B, Andersson M, Vilkki J, Toppari J, Kotaja N. Expression of SPEF2 during mouse spermatogenesis and identification of IFT20 as an interacting protein. Biol Reprod. 2010;82:580–90.
Zhang H, Mitchell DR. Cpc1, a Chlamydomonas central pair protein with an adenylate kinase domain. J Cell Sci. 2004;117:4179–88.
Dong F, Shinohara K, Botilde Y, Nabeshima R, Asai Y, Fukumoto A, et al. Pih1d3 is required for cytoplasmic preassembly of axonemal dynein in mouse sperm. J Cell Biol. 2014;204:203–13.
Tokuyasu KT. Dynamics of spermiogenesis in Drosophila melanogaster: 3. Relation between axoneme and mitochondrial derivatives. Exp Cell Res. 1974;84:239–50.
Otani H, Tanaka O, Kasai K, Yoshioka T. Development of mitochondrial helical sheath in the middle piece of the mouse spermatid tail: regular dispositions and synchronized changes. Anat Rec. 1988;222:26–33.
Ho HC, Wey S. Three dimensional rendering of the mitochondrial sheath morphogenesis during mouse spermiogenesis. Microsc Res Tech. 2007;70:719–23.
Bach D, Pich S, Soriano FX, Vega N, Baumgartner B, Oriola J, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. J Biol Chem. 2003;278:17190–7.
Hales KG, Fuller MT. Developmentally regulated mitochondrial fusion mediated by a conserved, novel, predicted GTPase. Cell. 1997;90:121–9.
Vadnais ML, Lin AM, Gerton GL. Mitochondrial fusion protein MFN2 interacts with the mitostatin-related protein MNS1 required for mouse sperm flagellar structure and function. Cilia. 2014;3:5.
Zhou J, Yang F, Leu NA, Wang PJ. MNS1 is essential for spermiogenesis and motile ciliary functions in mice. PLoS Genet. 2012;8:e1002516.
Sampson MJ, Ross L, Decker WK, Craigen WJ. A novel isoform of the mitochondrial outer membrane protein VDAC3 via alternative splicing of a 3-base exon. Functional characteristics and subcellular localization. J Biol Chem. 1998;273:30482–6.
Pedersen H, Mygind N. Absence of axonemal arms in nasal mucosa cilia in Kartagener’s syndrome. Nature. 1976;262:494–5.
Bleau G, Richer C-L, Bousquet D. Absence of dynein arms in cilia of endocervical cells in a fertile woman. Fertil Steril. 1978;30:362–3.
Jean Y, Langlais J, Roberts KD, Chapdelaine A, Bleau G. Fertility of a woman with nonfunctional ciliated cells in the Fallopian tubes. Fertil Steril. 1979;31:349–50.
Escudier E, Escalier D, Homasson J-P, Pinchon MC, Bernaudin JF. Unexpectedly normal cilia and normal spermatozoa in an infertile man with Kartagener’s syndrome. Eur J Respir Dis. 1987;70:180–3.
Olbrich H, Haffner K, Kispert A, Volkel A, Volz A, Sasmaz G, et al. Mutations in DNAH5 cause primary ciliary dyskinesia and randomization of left–right asymmetry. Nat Genet. 2002;30:143–4.
Supp DM, Witte DP, Potter SS, Brueckner M. Mutation of an axonemal dynein affects left–right asymmetry in inversus viscerum mice. Nature. 1997;389:963–6.
Zhang YJ, O’Neal WK, Randell SH, Blackburn K, Moyer MB, Boucher RC, et al. Identification of dynein heavy chain 7 as an inner arm component of human cilia that is synthesized but not assembled in a case of primary ciliary dyskinesia. J Biol Chem. 2002;277:17906–15.
Pennarun G, Escudier E, Chapelin C, Bridoux AM, Cacheux V, Roger G, et al. Loss-of-function mutations in a human gene related to Chlamydomonas reinhardtii dynein IC78 result in primary ciliary dyskinesia. Am J Hum Genet. 1999; 65:1508–19..
Loges NT, Olbrich H, Fenske L, Mussaffi H, Horvath J, Fliegauf M, et al. DNAI2 mutations cause primary ciliary dyskinesia with defects in the outer dynein arm. Am J Hum Genet. 2008;83:547–58.
Mazor M, Alkrinawi S, Chalifa-Caspi V, Manor E, Sheffield VC, Aviram M, et al. Primary ciliary dyskinesia caused by homozygous mutation in DNAL1, encoding dynein light chain 1. Am J Hum Genet. 2011;88:599–607.
Duriez B, Duquesnoy P, Escudier E, Bridoux AM, Escalier D, Rayet I, et al. A common variant in combination with a nonsense mutation in a member of the thioredoxin family causes primary ciliary dyskinesia. Proc Natl Acad Sci USA. 2007;104:3336–41.
Rashid S, Grzmil P, Drenckhahn JD, Meinhardt A, Adham I, Engel W, et al. Disruption of the murine dynein light chain gene Tcte3–3 results in asthenozoospermia. Reproduction. 2010;139:99–111.
Hjeij R, Onoufriadis A, Watson CM, Slagle CE, Klena NT, Dougherty GW, et al. CCDC151 mutations cause primary ciliary dyskinesia by disruption of the outer dynein arm docking complex formation. Am J Hum Genet. 2014;95:257–74.
Lechtreck KF, Witman GB. Chlamydomonas reinhardtii hydin is a central pair protein required for flagellar motility. J Cell Biol. 2007;176:473–82.
Sapiro R, Kostetskii I, Olds-Clarke P, Gerton GL, Radice GL, Strauss IJ. Male infertility, impaired sperm motility, and hydrocephalus in mice deficient in sperm-associated antigen 6. Mol Cell Biol. 2002;22:6298–305.
Teves ME, Zhang Z, Costanzo RM, Henderson SC, Corwin FD, Zweit J, et al. Sperm-associated antigen-17 gene is essential for motile cilia function and neonatal survival. Am J Respir Cell Mol Biol. 2013;48:765–72.
Fernandez-Gonzalez A, Kourembanas S, Wyatt TA, Mitsialis SA. Mutation of murine adenylate kinase 7 underlies a primary ciliary dyskinesia phenotype. Am J Respir Cell Mol Biol. 2009;40:305–13.
Takaki E, Fujimoto M, Nakahari T, Yonemura S, Miyata Y, Hayashida N, et al. Heat shock transcription factor 1 is required for maintenance of ciliary beating in mice. J Biol Chem. 2007;282:37285–92.
Tanaka H, Iguchi N, Toyama Y, Kitamura K, Takahashi T, Kaseda K, et al. Mice deficient in the axonemal protein Tektin-t exhibit male infertility and immotile-cilium syndrome due to impaired inner arm dynein function. Mol Cell Biol. 2004;24:7958–64.
Horani A, Ferkol TW, Shoseyov D, Wasserman MG, Oren YS, Kerem B, et al. LRRC6 mutation causes primary ciliary dyskinesia with dynein arm defects. PLoS One. 2013;8:e59436.
Moore DJ, Onoufriadis A, Shoemark A, Simpson MA, zur Lage PI, de Castro SC, et al. Mutations in ZMYND10, a gene essential for proper axonemal assembly of inner and outer dynein arms in humans and flies, cause primary ciliary dyskinesia. Am J Hum Genet. 2013;93:346–56.
Yanagisawa H, Kamiya R. A Tektin homologue is decreased in Chlamydomonas mutants lacking an axonemal inner-arm dynein. Mol Biol Cell. 2004;15:2105–15.
Acknowledgments
This work was supported in part by Grants-in-aid #15H01201 for Scientific Research on Innovative Areas and #22370023 for Scientific Research (B) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan (MEXT). Studies with marine invertebrates cited in this paper were supported by the members of the Onagawa Field Research Center, Tohoku University; International Coastal Research Center, AORI, The University of Tokyo; and staff of the National Bioresource Project.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Human/animal studies
This article does not contain any studies with human or animal subjects performed by any of the authors.
About this article
Cite this article
Inaba, K., Mizuno, K. Sperm dysfunction and ciliopathy. Reprod Med Biol 15, 77–94 (2016). https://doi.org/10.1007/s12522-015-0225-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12522-015-0225-5