Abstract
Background
Currently, coagulase negative staphylococci (CoNS) have got much attention as a serious health problem especially in neonates and children. High incidence of antibiotic resistance, in particular methicillin resistance, has complicated the treatment of these organisms. The aim of this study is to determine the susceptibility to different antimicrobial agents and the prevalence of macrolideslincosamides-streptogramins B (MLSB) resistance in CoNS isolates obtained from pediatric patients.
Methods
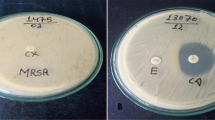
Totally 157 CoNS isolates from various clinical samples were examined for antibiotic resistance using disk diffusion and E-test methods. Double-disk test was applied to detect constitutive and inducible MLSB resistance (cMLSB and iMLSB) phenotypes.
Results
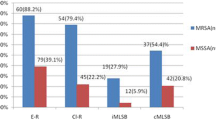
Resistance to methicillin was seen in 98 (62.4%) isolates. All isolates were susceptible to vancomycin and linezolid. The prevalence of resistance to antibiotics tested was as follows: fusidic acid (n=58, 36.9%), gentamicin (n=73, 46.5%), ciprofloxacin (n=81, 51.6%), clindamycin (n=112, 71.3%), erythromycin (n=129, 82.2%) and trimethoprim/sulfamethoxazole (n=133, 84.7%). iMLSB phenotype was seen in 14 (8.9%) isolates, and 18 (11.5%) and 98 (62.4%) isolates showed MS and cMLSB phenotypes, respectively. We observed that high overall antibiotic resistance rates were associated significantly with methicillin resistance. Conversely, iMLSB phenotype was correlated neither with methicillin resistance nor with invasiveness.
Conclusion
Given the similarity observed between the prevalence of iMLSB and MS phenotypes, the performance of disk diffusion induction test is strongly recommended in our region.
Similar content being viewed by others
References
Cheung GY, Otto M. Understanding the significance of Staphylococcus epidermidis bacteremia in babies and children. Curr Opin Infect Dis 2010;23:208–216.
Johnson JG, Saye EJ, Jimenez-Truque N, Soper N, Thomsen I, Talbot TR, et al. Frequency of Disinfectant Resistance Genes in Pediatric Strains of Methicillin-Resistant Staphylococcus aureus. Infect Control Hosp Epidemiol 2013;34:1326–1327.
Tamma PD, Robinson GL, Gerber JS, Newland JG, DeLisle CM, Zaoutis TE, et al. Pediatric Antimicrobial Susceptibility Trends across the United States. Infect Control Hosp Epidemiol 2013;34:1244–1251.
Ruppé E, Barbier F, Mesli Y, Maiga A, Cojocaru R, Benkhalfat M, et al. Diversity of Staphylococcal Cassette Chromosome mec Structures in Methicillin-Resistant Staphylococcus epidermidis and Staphylococcus haemolyticus Strains among Outpatients from Four Countries. Antimicrob Agents Chemother 2009;53:442–449.
Otto M. Staphylococcus epidermidis: the’ accidental’ pathogen. Nat Rev Microbiol 2009;7:555–567.
Delialioglu N, Aslan G, Ozturk C, Baki V, Sen S, Emekdas G. Inducible clindamycin resistance in staphylococci isolated from clinical samples. Jpn J Infect Dis 2005;58:104–106.
Fiebelkorn KR, Crawford SA, McElmeel ML, Jorgensen JH. Practical disk diffusion method for detection of inducible clindamycin resistance in Staphylococcus aureus and coagulasenegative staphylococci. J Clin Microbiol 2003;41:4740–4744.
Lewis JS, Jorgensen JH. Inducible clindamycin resistance in Staphylococci: should clinicians and microbiologists be concerned? Clin Infect Dis 2005;40:280–285.
Stoll BJ, Hansen N, Fanaroff AA, Wright LL, Carlo WA, Ehrenkranz RA, et al. Late-onset sepsis in very low birth weight neonates: the experience of the NICHD Neonatal Research Network. Pediatrics 2002;110:285–291.
Keim LS, Torres-Filho SR, Silva PV, Teixeira LA. Prevalence, aetiology and antibiotic resistance profiles of coagulase negative staphylococci isolated in a teaching hospital. Braz J Microbiol 2011;42:248–255.
Mombach-Pinheiro-Machado AB, Reiter KC, Paiva RM, Barth AL. Distribution of staphylococcal cassette chromosome mec (SCCmec) types I, II, III and IV in coagulase-negative staphylococci from patients attending a tertiary hospital in southern Brazil. J Med Microbiol 2007;56:1328–1333.
Kozitskaya S, Olson ME, Fey PD, Witte W, Ohlsen K, Ziebuhr W. Clonal Analysis of Staphylococcus epidermidis Isolates Carrying or Lacking Biofilm-Mediating Genes by Multilocus Sequence Typing. J Clin Microbiol 2005;43:4751–4757.
Performance standards for antimicrobial susceptibility testing; 21st informational supplement. CLSI document M100-S21. Clinical and Laboratory Standards Institute, 2011.
Piette A, Verschraegen G. Role of coagulase-negative staphylococci in human disease. Vet Microbiol 2009;134:45–54.
Mert G, Kiliç A, Bedir O, Basustaoglu AC. Clinical significance and staphylococcal cassette chromosome mec (SCCmec) characterization of coagulase-negative staphylococci isolated from blood cultures. Turk J Med Sci 2011;41:859–865.
Koksal F, Yasar H, Samasti M. Antibiotic resistance patterns of coagulase-negative staphylococcus strains isolated from blood cultures of septicemic patients in Turkey. Microbiol Res 2009;164:404–410.
Otto M. Virulence factors of the coagulase-negative staphylococci. Front Biosci 2004;9:841–863.
Mamishi S, Pourakbari B, Ashtiani MH, Hashemi FB. Frequency of isolation and antimicrobial susceptibility of bacteria isolated from bloodstream infections at Children’s Medical Center, Tehran, Iran, 1996–2000. Int J Antimicrob Agents 2005;26:373–379.
Javadpour S, Karimi E, Karmostaji A. Frequency and antibiogram pattern of coagulase negative Staphylococcus in clinical specimens of Shahid Mohammadi Hospital in patients, Bandar-Abbas, Iran. Afr J Microbiol Res 2010;4:1581–1583.
Klingenberg C, Sundsfjord A, Rønnestad A, Mikalsen J, Gaustad P, Flaegstad T. Phenotypic and genotypic aminoglycoside resistance in blood culture isolates of coagulase-negative staphylococci from a single neonatal intensive care unit, 1989–2000. J Antimicrob Chemother 2004;54:889–896.
Castanheira M, Watters AA, Bell JM, Turnidge JD, Jones RN. Fusidic acid resistance rates and prevalence of resistance mechanisms among Staphylococcus spp. isolated in North America and Australia, 2007–2008. Antimicrob Agents Chemother 2010;54:3614–3617.
Deveci Ö, Kiliç D, Kaygusuz S, Duruyürek N, Karabiçak C, Agalar C, et al. Evaluation of resistance to fusidic acid in Staphylococci isolates. J Microbiol Infect Dis 2011;1:22–25.
Eksi F, Gayyurhan ED, Bayram A, Karsligil T. Determination of antimicrobial susceptibility patterns and inducible clindamycin resistance in Staphylococcus aureus strains recovered from southeastern Turkey. J Microbiol Immunol Infect 2011;44:57–62.
Schreckenberger PC, Ilendo E, Ristow KL. Incidence of constitutive and inducible clindamycin resistance in Staphylococcus aureus and coagulase-negative staphylococci in a community and a tertiary care hospital. J Clin Microbiol 2004;42:2777–2779.
Perez LR, Caierão J, Antunes AL, D’Azevedo PA. Use of the D test method to detect inducible clindamycin resistance in coagulase negative staphylococci (CoNS). Braz J Infect Dis 2007;11:186–188.
Azap OK, Arslan H, Timurkaynak F, Yapar G, Oruç E, Gagir U. Incidence of inducible clindamycin resistance in staphylococci: first results from Turkey. Clin Microbiol Infect 2005;11:582–584.
Yilmaz G, Aydin K, Iskender S, Caylan R, Koksal I. Detection and prevalence of inducible clindamycin resistance in staphylococci. J Med Microbiol 2007;56:342–345.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Aghazadeh, M., Ghotaslou, R., Ahangarzadeh Rezaee, M. et al. Determination of antimicrobial resistance profile and inducible clindamycin resistance of coagulase negative staphylococci in pediatric patients: the first report from Iran. World J Pediatr 11, 250–254 (2015). https://doi.org/10.1007/s12519-014-0524-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12519-014-0524-7