Abstract
Background
Staphylococcus aureus is a global public health issue in both community and hospital settings. Management of methicillin-resistant S. aureus (MRSA) infections are tough owing to its resistance to many antibiotics. Macrolide-lincosamide-streptogramin B (MLSB) antibiotics are commonly used for the management of MRSA. This study was aimed to determine the occurrence of inducible clindamycin- and methicillin-resistant S. aureus at a tertiary care hospital in Kathmandu, Nepal.
Methods
A total of 1027 clinical samples were processed following standard laboratory procedures and antibiotic susceptibility testing of S. aureus was performed by disc diffusion method. MRSA isolates were detected phenotypically using cefoxitin disc, and inducible clindamycin resistance was detected phenotypically using the D-zone test.
Results
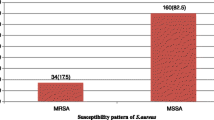
Of 1027 samples, 321 (31.2%) were culture positive, of which 38 (11.8%) were S. aureus. All S. aureus isolates were susceptible to vancomycin, and 25 (67%) of S. aureus isolates were multidrug-resistant. Similarly, 15 (39.5%) of S. aureus were MRSA and 14 (36.5%) were inducible clindamycin-resistant phenotypes.
Conclusion
Inducible clindamycin and methicillin resistance were common in S. aureus. This emphasizes that the methicillin resistance test and the D-zone test should be incorporated into the routine antibiotic susceptibility testing in hospital settings.
Similar content being viewed by others
Introduction
Globally, Staphylococcus aureus is a leading cause of nosocomial and community-acquired infections [1]. The rampant use of antibiotics has increased the selective pressure on bacteria, resulting in the emergence of drug resistance. These resistant bacteria pose a global public health threat [2]. The dissemination and spread of resistant isolates reduce the efficacy of antimicrobial agents which in turn prolongs hospital stays, increases treatment costs, and increases fatalities [3]. One of the common resistance mechanisms in S. aureus is methicillin resistance. The methicillin resistance was first reported in 1961, just 2 years after the first clinical use of methicillin [4]. Since then, the rapid rise of methicillin-resistant S. aureus (MRSA) has limited the therapeutic choices for the management of MRSA infections [5].
Antibiotics like vancomycin, linezolid, quinupristin, and dalfopristin have long been the preferred choice for the management of such MRSA isolates. However, the increasing reports of resistance against these antibiotics have only increased the skepticism on their efficacy [6, 7]. This suspicion has led clinicians to choose the macrolide lincosamide-streptogramin B (MLSB) family of antibiotics, a reserved alternative, for the management of MRSA isolates. In the MLSB family, a commonly used clindamycin is an ideal antibiotic owing to its excellent pharmacokinetics [8]. With time and overuse, S. aureus is acquiring resistance against MLSB too. Resistance against MSLB antibiotics can be either constitutive or inducible. The constitutive resistance mechanism is mediated through msrA genes, in which S. aureus strains are resistant to erythromycin and sensitive to clindamycin, in both in vivo and in vitro. The constitutively resistant strains do not develop clindamycin resistance during therapy [9, 10]. The inducible MLSB (iMLSB) resistant isolates show resistance against erythromycin but are susceptible to clindamycin. In the presence of a powerful methylase enzyme inducer like erythromycin, iMLSB resistance develops. Unlike constitutive MLSB (cMLSB) resistance, iMLSB resistance cannot be detected by standard susceptibility testing. The inducible clindamycin resistance can be detected by the D-zone test, i.e. D-shaped distorted inhibition zone around clindamycin under the in-vitro effect of erythromycin [11]. It is critical to identify the iMLSB resistance for the proper management of S. aureus [12]. Otherwise, clindamycin administration can lead to treatment failure by the development of constitutive resistance [13].
The prevalence of methicillin and inducible clindamycin resistance among S. aureus varies considerably as per settings and regions. Various studies have reported the prevalence of MRSA in the world ranging from 20 to 58% [9, 11, 12, 14,15,16,17,18,19]. Few studies have reported MRSA in Nepal ranging from 25 to 64% [20,21,22,23,24,25]. Similarly, various studies had reported prevalence of inducible clindamycin resistance among S. aureus in the world ranging from 7 to 34% [1, 9,10,11,12, 14,15,16,17,18,19]. Few studies have reported inducible clindamycin resistance among S. aureus in Nepal ranging from 11 to 40% [20,21,22,23,24,25,26]. However, it is largely under-reported as there are limited studies on the detection of methicillin and inducible clindamycin resistance in S. aureus. Ironically, the detection of methicillin and inducible clindamycin resistance are still not a part of routine susceptibility investigations of S. aureus in hospital settings. The local resistance data is crucial for optimizing antibiotics usage, guiding empirical treatment, and managing infection effectively. The study aimed to explore the burden of methicillin and inducible clindamycin resistance among S. aureus in a tertiary care hospital in Nepal. Since antimicrobial susceptibility among pathogens is dynamics of time and space, findings from such surveillance study will assist clinicians of the region in the jurisdiction of appropriate antibiotics and improve clinical management of infections.
Methods
Study design, study area, and sample population
This cross-sectional study was carried out from August 2018 to March 2019 at Kantipur Hospital in Kathmandu, Nepal. The hospital is a 100-bed referral hospital located at the Eastern gate of the capital city, Kathmandu. The hospital serves patients of the Kathmandu valley and other nearby areas. Also, the hospital serves patients referred to from other hospitals outside the Kathmandu valley, especially those from Eastern region of the country. Kathmandu has a total area of 50.67 sq. km and has an average elevation of 1400 m above sea level. The population density is approximately 4416 per sq. km. The sample population included patients of all age groups and genders visiting the hospital during the study period with symptoms of suspected infections. Both inpatients and outpatients were included in the study. The demographic information of the patients was retrieved from the medical records of the hospital. The samples from the patients were collected following the physician’s clinical diagnosis. A total of 1027 clinical samples were included in the study. The samples included urine (n = 859), pus (n = 52), blood (n = 50), sputum (n = 41), and body fluids (n = 25; CSF, synovial fluid, pleural fluid, throat swabs, vaginal swabs). Repeated samples and samples showing the possible signs of contaminations were excluded.
Sample processing and identification of S. aureus
All the samples were cultured in a routine culture media following the standard microbiological protocols. In brief, the samples were first streaked on blood agar and mannitol salt agar. Then, the mannitol salt agar plates were incubated aerobically at 37 °C for 24 h, while blood agar plates were incubated in a candle jar at 37 °C for 24 h. Plates yielding cream to golden yellow colonies with or without hemolysis on blood agar, and yellow colonies on mannitol salt agar were subcultured in nutrient agar [27, 28]. S. aureus isolates were identified based on growth in culture, Gram’s staining reactions (Gram-positive cocci in a cluster), and various biochemical properties (catalase-positive, coagulase-positive, DNase-positive, nitrate reduction-positive, and glucose OF-fermentative) [28].
Antibiotic susceptibility testing
All identified isolates of S. aureus were tested for susceptibility against the commercially available antibiotic discs by modified Kirby-Bauer disc diffusion method on Mueller–Hinton agar (MHA) (HiMedia Pvt. Ltd., India), as described in CLSI M100-S28 [29]. In brief, 0.5 McFarland bacterial suspensions were prepared from a single isolated colony on the nutrient broth. Lawn cultures of this bacterial suspension were performed over the MHA plate. Subsequently, antibiotic discs were placed on the agar surface using sterile forceps. Finally, the plates were incubated aerobically at 37 °C for 24 h [29]. The antibiotic discs tested were amikacin (30 μg), ampicillin (10 μg), cefoxitin (30 μg), ceftriaxone (30 μg), ciprofloxacin (5 μg), clindamycin (2 μg), co-trimoxazole (sulpha/trimethoprim) (23.75/ 1.25 μg), erythromycin (15 μg), gentamicin (10 μg), norfloxacin (10 μg), oxacillin (1 μg), penicillin-G (10 μg), tetracycline (30 μg), vancomycin (Etest for MIC) (HiMedia Pvt. Ltd., India). The zone diameters were interpreted as per CLSI M100-S28 [29]. The CLSI recommends interpreting antibiotic susceptibility results as resistant (R), or intermediate (I), or susceptible (S), but we dichotomously categorized them as susceptible (S) or resistant (R). The isolates showing intermediate susceptibility were also considered resistant. Quality control was maintained using S. aureus ATCC 25923. The tested antibiotics were categorized into nine classes viz., aminoglycoside, cephems, fluoroquinolone, folate pathway inhibitor, glycopeptide, lincosamide, macrolide, penicillin, and tetracycline. S. aureus resistant to three or more different classes of antibiotics were considered multidrug-resistant (MDR) [30].
Phenotypic detection of MRSA
The MRSA isolates were confirmed phenotypically using cefoxitin disc (30 μg) (HiMedia Pvt. Ltd., India). As recommended by CLSI M100-S28, cefoxitin disc was used as a surrogate for detection of mecA-mediated oxacillin resistance, i.e. MRSA, by disc diffusion method. The plates were incubated aerobically at 33 to 35 °C for 18 h S. aureus yielding zone diameter of 21 mm or less with cefoxitin disc was phenotypically confirmed as MRSA, as per CLSI M100-S28 [29]. S. aureus ATCC 25923 was used as quality control.
Phenotypic detection of inducible clindamycin resistance (iMLSB phenotypes)
The S. aureus showing resistance to erythromycin was further tested for inducible resistance to clindamycin. This was tested by the D-zone test as described in CLSI M100-S28 [29]. Briefly, 0.5 McFarland standard bacterial suspension of S. aureus was lawn cultured over the MHA plate (HiMedia Pvt. Ltd., India). Then, the erythromycin disc (15 μg) (HiMedia Pvt. Ltd., India) was placed at a distance of 15 mm from the clindamycin disc (2 μg) (HiMedia Pvt. Ltd., India). The plates were incubated aerobically at 37 °C for 18–24 h. The induction test results were interpreted as three different phenotypes of S. aureus. The moderate sensitive (MS) phenotypes, if isolates were resistant to erythromycin (zone diameter ≤ 13 mm) and susceptible to clindamycin (zone diameter ≥ 21 mm) without D-shaped zone. The iMLSB phenotypes, if isolates were resistant to erythromycin (zone diameter ≤ 13 mm) and susceptible to clindamycin (zone diameter ≥ 21 mm) with a D-shaped zone. (Fig. 1B) The cMLSB phenotypes, if isolates were resistant to both erythromycin (zone diameter ≤ 13 mm) and clindamycin (zone diameter ≤ 14 mm) [29]. S. aureus ATCC 25923 was used as quality control. Separate in-house S. aureus isolates that were confirmed as the clindamycin resistance phenotypes were also used for quality control.
Statistical analysis
All the generated data were entered and curated by using Microsoft Excel® version 2016 (Microsoft Corporation, USA). All statistical analyses were done in R software© version 4.1.1 (R Core Team, Austria). Descriptive summaries were presented in text and tables. Descriptive statistics were expressed as percentages. The 2 × 2 contingency tables were constructed for the categorical variables and the contingency or association was tested using the chi-square test. The Yates correction was applied in the chi-square test whenever applicable. Pearson’s Phi- coefficient was used to measure the effect size, i.e. the strength of the association between two variables. Cohen’s rules-of-thumb were used to interpret the Phi-coefficient. The p-value of less than 0.05 was considered statistically significant whenever applicable.
Results
Of 1027 samples, 321 (31.2%) samples were culture positive. Of 321 culture positives, 82 (25.5%) were Gram-positive, and, 38 (46.4%) were S. aureus. (Table 1).
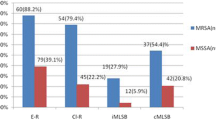
In the induction test, 14 (36.5%) S. aureus were iMLSB phenotypes and 7 (18.5%) were cMLSB phenotypes. The cMLSB and iMLSB phenotypes were nearly similar in MRSA and methicillin-susceptible Staphylococcus aureus (MSSA) phenotypes. There was no significant association between cMLSB phenotypes and methicillin resistance in S. aureus (chi-square with Yates correction (df = 1, N = 38) = 0.05, p = 0.822). Also, there was a positive, but a very weak effect size (Phi-coefficient = 0.04). Similarly, there was no significant association between iMLSB phenotypes and methicillin resistance in S. aureus (chi-square (df = 1, N = 38) = 0.11, p = 0.745). Also, there was a positive but a very weak effect size (Phi-coefficient = 0.05). (Table 3).
Discussion
S. aureus are one of the most common skin colonizing bacteria, and are a leading source of nosocomial and community-acquired skin infections. Of 1027 samples, 11.8% yielded S. aureus. S. aureus was higher in pus samples (39.2%). S. aureus is normally found in the environment and skin surface, so it is common in wound swabs and pus samples.
In susceptibility testing, all 38 isolates of S. aureus were sensitive to vancomycin. All 38 isolates of S. aureus were resistant to penicillin-G and ampicillin. Out of 38 S. aureus, 67.5% were MDR, and 50% were MRSA. Similar findings were reported by studies in Nepal [21, 23]. Higher percentages were reported by studies in Nepal [14, 22, 24, 26] while lower percentages were reported by other studies in Nepal [20, 25]. The emergence of resistance to multiple antibiotics among S. aureus is impeding their effective management. This persuades the physicians to opt for the use of reserve drugs, like MLSB family. Lower cost, lower side effects, and better tissue penetration make clindamycin a better choice among MLSB family. Clindamycin has been used for the treatment of severe staphylococcal infections, like MRSA. During clindamycin therapy, the iMLSB strains can gradually develop constitutively resistant mutants both, in vitro and in vivo. This leads to treatment failures in certain patients. Hence, the detection of such resistant phenotypes is important to minimize treatment failure. S. aureus resistance to macrolides may be constitutive or inducible clindamycin resistance, or may solely be macrolides-resistant [10]. However, among erythromycin-resistant S. aureus isolates there has been a rise in inducible clindamycin resistance [18]. In this study, about a third of S. aureus (36.5%) were iMLSB phenotypes. Similar findings were reported by studies in Nepal [14, 26]. However, lower percentages were reported by other studies in Nepal [20,21,22,23,24,25] and elsewhere [15,16,17,18]. Likewise, 18.5% of S. aureus were cMLSB phenotypes. Similar findings were reported by different studies in Nepal [14, 26] and elsewhere [15, 16]. However, lower percentages were reported by different studies in Nepal [24, 26]. Higher percentages were reported by different studies in Nepal [20, 22] and elsewhere [17, 18]. Also, 18.5% of S. aureus were MS phenotypes. Similar findings were reported by studies in Nepal [20, 23] while higher percentages were reported by other studies in Nepal [23, 26]. These variances in the reporting of MLSB resistance among S. aureus might be related to changes in circulating clones, as well as disparities in infection prevention measures and antibiotic prescribing trends in different hospital settings. Also, the prevalence of MLSB antibiotic-resistant phenotypes varies based on the geography and the characteristics of subjects, like inpatients or outpatients, hospital or community origin, children or adults, public or private institutions, patients or healthcare workers. This emphasizes the need for surveillance programs at nation, region, or hospital level [15,16,17, 20].
The reporting of MRSA has dramatically risen in recent years. However, there is a stark variation in its reporting among the countries. Inadequate infection-prevention practices in hospitals, indiscriminate antibiotic use, intravascular catheterization, hospitalization in intensive care units, and other factors all contribute to MRSA rise [31]. In this study, 39.4% of S. aureus isolates were MRSA. Similar findings were reported by studies in Nepal [21] and elsewhere [17, 18]. However, higher percentages were reported by other studies in Nepal [22, 23, 26] and elsewhere [11, 14]. And, lower percentages were reported by other studies in Nepal [12, 20, 25] and elsewhere [15, 16]. These variances in MRSA reporting among S. aureus isolates might be related to changes in circulating clones in a different geography, as well as disparities in infection- prevention measures and antibiotic prescribing trends in different hospital settings.
In this study, there was no substantively significant association between cMLSB resistance and methicillin resistance in S. aureus (chi-square with Yates correction (df = 1, N = 38) = 0.05, p = 0.822, Phi-coefficient = 0.04). Also, there was no substantively significant association between iMLSB resistance and methicillin resistance in S. aureus (chi-square (df = 1, N = 38) = 0.11, p = 0.745, Phi-coefficient = 0.06). Genotyping i.e., detection of the erm genes is considered a superior tool for surveillance of MLSB resistance. But the continuous mutations in erm genes make the use of genotyping tools difficult. Also, the use of such expensive tools in poor resource settings is hypothetical. In contrast, the phenotypic techniques indicate both the presence and expression of the responsible erm gene. The D-zone test is a simple and cheap phenotypic technique using erythromycin and clindamycin discs. This is a phenotypic disc diffusion test recommended by CLSI [29]. The D-zone test relies on the ability of erythromycin to induce resistance against clindamycin. A flattening of the zone of inhibition around the clindamycin disc in the proximity of the erythromycin disc, producing a D-shaped zone of inhibition, is considered a positive D-zone result. This indicates the induction of clindamycin resistance by erythromycin. The D-zone test has a high throughput reporting different types of phenotypic resistance in a single test. This method has a sensitivity of 100% when the distance of two test disk is 15 mm [32].
Globally, AMR is on the rise, particularly in developing countries, like Nepal. Over the counter sale of antibiotics, lack of effective regulations on antibiotics use, incomplete dosing, excessive use of wide-spectrum antibiotics for common infections, and empiric therapy without laboratory diagnosis are all common in developing countries like Nepal. These practices usually cure infections, so most health settings opt for and retain these practices, but in return, these settings act as a factory of resistant mutants. This, in part, is because of a lack of sufficient resources to set up standard laboratory facilities covering all geography, particularly in remote skirts of developing countries like Nepal. AMR is a public health threat that demands urgent attention. Surveillance of this type reports the updated AMR profile of the circulating pathogens in the region, which in turn can be used for formulating policies with strong strategies to check AMR.
Limitations
First, the selection bias could have accounted for some errors in the findings. Second, the study was conducted for six months and the total samples were 1027 in a single setting. This is a comparatively small sample size over the shorter duration. Thus, the generalization of the findings may not be accurate. However, we believe that the medical practices and patient distributions in every hospital in the capital city, Kathmandu, are more or less similar, excluding the specialized hospitals. Thus, we believe our findings represent the population scenario of the region. Third, the study design consisted only of the phenotypic characterization, which could be the result of many intrinsic and extrinsic factors. The genotypic characterization could have provided further clear insights.
Conclusions
The high proportions of MRSA and iMLSB phenotypes among S. aureus emphasize the need for methicillin resistance test and D-test to be incorporated in routine susceptibility testing for effective management of S. aureus. This also warrant the need for larger epidemiological AMR surveillances and policy updates to manage S. aureus effectively.
Availability of data and materials
The complete dataset generated and analyzed during the study is already covered in the text. The raw data can be made available at reasonable request to the corresponding author.
Abbreviations
- CD:
-
Clindamycin
- CLSI:
-
Clinical and Laboratory Standards Institute
- cMLSB:
-
Constitutive MLSB phenotype
- D− :
-
D-zone test negative
- D+ :
-
D-zone test positive
- EM:
-
Erythromycin
- iMLSB:
-
Inducible MLSB phenotype
- MDR:
-
Multidrug-resistant
- MHA:
-
Mueller–Hinton agar
- MLSB:
-
Macrolide lincosamide-streptogramin B family of antibiotics
- MRSA:
-
Methicillin-resistant Staphylococcus aureus
- MS:
-
Macrolide-streptogramin B phenotype
- MSSA:
-
Methicillin-susceptible Staphylococcus aureus
- R:
-
Resistant
- S:
-
Susceptible
References
Goudarzi M, Kobayashi N, Dadashi M, Pantůček R, Nasiri MJ, Fazeli M. Prevalence, genetic diversity, and temporary shifts of inducible clindamycin resistance Staphylococcus aureus Clones in Tehran, Iran: a molecular–epidemiological analysis from 2013 to 2018. Front Microbiol. 2020. https://doi.org/10.3389/fmicb.2020.00663.
Coates R, Moran J, Horsburgh MJ. Staphylococci: colonizers and pathogens of human skin. Future Microbiol. 2014;9(1):75–91.
World Health Organization (WHO). Antimicrobial resistance 2021. https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance. Accessed 13 Oct 2020.
Jevons MP. “Celbenin”—resistant Staphylococci. BMJ. 1961;1(5219):124–5.
Appelbaum PC. Microbiology of antibiotic resistance in Staphylococcus aureus. Clin Infect Dis. 2007;45(Supplement 3):S165–70.
Cong Y, Yang S, Rao X. Vancomycin resistant Staphylococcus aureus infections: a review of case updating and clinical features. J Adv Res. 2020;21:169–76.
Saravolatz LD, Eliopoulos GM. Quinupristin-dalfopristin and linezolid: evidence and opinion. Clin Infect Dis. 2003;36(4):473–81.
Gemmell CG, Edwards DI, Fraise AP, Gould FK, Ridgway GL, Warren RE, et al. Guidelines for the prophylaxis and treatment of methicillin-resistant Staphylococcus aureus (MRSA) infections in the UK. J Antimicrob Chemother. 2006;57(4):589–608.
Lim HS, Lee H, Roh KH, Yum JH, Yong D, Lee K, et al. Prevalence of inducible clindamycin resistance in staphylococcal isolates at a Korean Tertiary Care Hospital. Yonsei Med J. 2006;47(4):480–4.
Deotale V, Mendiratta DK, Raut U, Narang P. Inducible clindamycin resistance in Staphylococcus aureus isolated from clinical samples. Indian J Med Microbiol. 2010;28(2):124–6.
Saffar H, Rajabiani A, Abdollahi A, Habibi S, Baseri Z. Frequency of inducible clindamycin resistance among gram-positive cocci in a tertiary hospital, Tehran, Iran. Iran J Microbiol. 2016;8(4):243–8.
Khashei R, Malekzadegan Y, Sedigh Ebrahim-Saraie H, Razavi Z. Phenotypic and genotypic characterization of macrolide, lincosamide and streptogramin B resistance among clinical isolates of staphylococci in southwest of Iran. BMC Res Notes. 2018;11(1):711.
Drinkovic D, Fuller ER, Shore KP, Holland DJ, Ellis-Pegler R. Clindamycin treatment of Staphylococcus aureus expressing inducible clindamycin resistance. J Antimicrob Chemother. 2001;48(2):315–6.
Shidiki A, Pandit BR, Vyas A. Characterization and prevalence of clindamycin resistance Staphylococcus aureus from clinical samples of National Medical College and Teaching Hospital, Nepal. Asian J Pharm Clin Res. 2019;12(5):90–2.
Bottega A, Rodrigues Mde A, Carvalho FA, Wagner TF, Leal IA, Santos SO, et al. Evaluation of constitutive and inducible resistance to clindamycin in clinical samples of Staphylococcus aureus from a tertiary hospital. Rev Soc Bras Med Trop. 2014;47(5):589–92.
Rahbar M, Hajia M. Inducible clindamycin resistance in Staphylococcus aureus: a cross-sectional report. PJBS. 2007;10(1):189–92.
Seifi N, Kahani N, Askari E, Mahdipour S, Naderi NM. Inducible clindamycin resistance in Staphylococcus aureus isolates recovered from Mashhad. Iran Iran J Microbiol. 2012;4(2):82–6.
Shetty J, Afroz Z. Prevalence of constitutive and inducible clindamycin resistance among clinical isolates of Staphylococcus aureus in a tertiary care institute in North India. Int J Res Med Sci. 2017;5(7):3120–5.
Baiu SH, Al-Abdli NE. Inducible clindamycin resistance in methicillin resistant Staphylococcus aureus. Am J Infect Dis Microbiol. 2016;4(1):25–7.
Adhikari RP, Shrestha S, Barakoti A, Amatya R. Inducible clindamycin and methicillin resistant Staphylococcus aureus in a tertiary care hospital, Kathmandu. Nepal BMC Infectious Diseases. 2017;17(1):483.
Ansari S, Nepal HP, Gautam R, Rayamajhi N, Shrestha S, Upadhyay G, et al. Threat of drug resistant Staphylococcus aureus to health in Nepal. BMC Infect Dis. 2014;14(1):157.
Mohapatra TM, Shrestha B, Pokhrel BM. Constitutive and inducible clindamycin resistance in Staphylococcus aureus and their association with meticillin-resistant S. aureus (MRSA): experience from a tertiary care hospital in Nepal. Int J Antimicrob Agents. 2009;33(2):187–9.
Nagarkoti D, Prajapati K, Sharma AN, Gyawali A, Manandhar S. Distribution of macrolide-lincosamide-streptogramin b antibiotics resistance genes in clinical isolates of staphylococci. J Nepal Health Res Counc. 2021;18(4):734–40.
Sah P, Khanal R, Lamichhane P, Upadhaya S, Lamsal A, Pahwa VK. Inducible and constitutive clindamycin resistance in Staphylococcus aureus: an experience from Western Nepal. Int J Biomed Res. 2015;6(5):316–9.
Timsina R, Shrestha U, Singh A, Timalsina B. Inducible clindamycin resistance and erm genes in Staphylococcus aureus in school children in Kathmandu Nepal. Future Sci OA. 2021. https://doi.org/10.2144/fsoa-2020-0092.
Regmi R, Khadka S, Sapkota S, Magar S, Adhikari S, Subedi S, et al. Phenotypic detection of inducible clindamycin resistance among clinical isolates of Staphylococcus aureus in Bharatpur Hospital. JCMS-Nepal. 2020;16(3):178–83.
Vandepitte J, Engbaek K, Rohner P, Piot P, Heuck CC, World Health Organization (WHO). Basic laboratory procedures in clinical bacteriology. 2nd ed. Geneva: World Health Organization; 2003.
Versalovic J, Carroll KC, Funke G, Jorgensen JH, Landry ML, Warnock DW. Manual of clinical microbiology. 10th ed. Washington: ASM Press; 2011.
Clinical and Laboratory Standards Institute (CLSI). Performance standards for antimicrobial susceptibility testing, in twenty-eighth informational supplement (M100–S28). Wayne: Clinical and Laboratory Standards Institute; 2018.
Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect. 2012;18(3):268–81.
Catry B, Latour K, Jans B, Vandendriessche S, Preal R, Mertens K, et al. Risk factors for methicillin resistant Staphylococcus aureus: a multi-laboratory study. PLoS ONE. 2014;9(2):e89579.
O’Sullivan MVN, Cai Y, Kong F, Zeng X, Gilbert GL. Influence of disk separation distance on accuracy of the disk approximation test for detection of inducible clindamycin resistance in Staphylococcus spp. J Clin Microbiol. 2006;44(11):4072–6.
Acknowledgements
We are indebted to the Department of Microbiology, Kantipur Hospital, Tinkune, Kathmandu, Nepal for the kind cooperation and support.
Funding
None.
Author information
Authors and Affiliations
Contributions
DT and DS conceptualized and designed the study methodology. DT performed the laboratory investigations. SP, ST, and SL collected data, analyzed data, reviewed the literature, prepared and edited the draft of the manuscript. MC and NA supervised the entire project and edited the manuscript. DS helped with data analysis and prepared the final version of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Ethics approval and consent to participate
All the samples and isolates were obtained as a part of a routine clinical investigation. Informed consent was not required, as the study did not interrupt patients’ routine clinical care. The written permission was obtained from the hospital for the further investigation of the isolates in the hospital setting, and the retrieval of the patients’ demographic data from medical records. The anonymity of the patients’ data was maintained throughout the study and data publication. All the procedures in the study were on par with relevant ethical standards and with the 1964 Helsinki Declaration, and its later amendments.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Thapa, D., Pyakurel, S., Thapa, S. et al. Staphylococcus aureus with inducible clindamycin resistance and methicillin resistance in a tertiary hospital in Nepal. Trop Med Health 49, 99 (2021). https://doi.org/10.1186/s41182-021-00392-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1186/s41182-021-00392-2