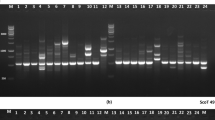
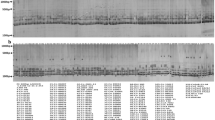
Abstract
Sugarcane is a main bioenergy crop and is highlighted worldwide in sugar, ethanol, and biomass production. Sugar and lignin contents are important quality traits for traditional and energy cane cultivar development, respectively. In the present study, genetic variability of a broad group of sugarcane basic germplasm accessions encompassing wild relatives and traditional and modern cultivars was assessed using target region amplification polymorphism (TRAP) markers derived from candidate genes involved in sugar and lignin metabolism. In total, 823 polymorphic markers (483 for sugar and 340 for lignin metabolism genes) were amplified; the average polymorphism information content values were highest for sugarcane wild relatives followed by traditional cultivars and modern cultivars. Genetic variability of the 96 genotypes captured by TRAP candidate genes for sugar and lignin metabolism was structured into two and three subpopulations, respectively. Based on the membership proportion (Q), modern cultivars inherited variability for genes involved in sugar metabolism from both S. officinarum and S. spontaneum. The genetic differentiation index based on sugar and lignin metabolism genes suggests moderate genetic differentiation among wild relatives, traditional cultivars, and modern cultivars. A core collection was established for sugar and lignin TRAP markers. Values for average genetic distance for the core collection based on sugar (0.761) and lignin (0.804) TRAP-derived markers were higher than those observed for all accessions, indicating that the core collections retained the most divergent accessions.





Similar content being viewed by others
References
Abuzayed, M., N. El-Dabba, A. Frary, and S. Doganlar. 2017. GDdom: An Online Tool for Calculation of Dominant Marker Gene Diversity. Biochemical Genetics 55: 155–157.
Aitken, K., and M. McNeill. 2010. Diversity Analysis. In Genetics, Genomics and Breeding of Sugarcane, ed. R. Henry and C. Kole, 19–42. Enfield: Science Publishers.
Aitken, K., J. Li, G. Piperidis, C. Qing, F. Yuanhong, and P. Jackson. 2018. Worldwide Genetic Diversity of the Wild Species Saccharum spontaneum and Level of Diversity Captured Within Sugarcane Breeding Programs. Crop Science 58(1): 218–229.
Alwala, S., A. Suman, J.A. Arro, J.C. Vermis, and C.A. Kimbeng. 2006. Target Region Amplification Polymorphism (TRAP) for Accessing Genetic Diversity in Sugarcane Germplasm Collections. Crop Science 46(1): 448–455.
Alwala, S., C. Kimbeng, J.C. Veremis, and K.A. Gravois. 2008. Linkage Mapping and Genome Analysis in a Saccharum Interspecific Cross Using AFLP SRAP and TRAP Markers. Euphytica 164(1): 37–51.
Amalraj, A., and N. Balasundaram. 2006. On the Taxonomy of the Members of ‘Saccharum Complex’. Genetic Resources and Crop Evolution 53(1): 35–41.
Arceneaux, G. 1965. Cultivated Sugarcane of the World and Their Botanical Derivation. Proceedings of the International Society of Sugar Cane Technologists 12: 844–854.
Brown, J.S., R.J. Schnell, E.J. Power, S.L. Douglas, and D.N. Kuhn. 2007. Analysis of Clonal Germplasm from Five Saccharum Species: S. barberi, S. robustum, S. officinarum, S. sinense and S. spontaneum: A Study of Inter- and Intra Species Relationships Using Microsatellite Markers. Genetic Resources and Crop Evolution 54(3): 627–648.
Cesarino, I., P. Araújo, A.P. Domingues Júnior, and P. Mazzafera. 2012. An Overview of Lignin Metabolism and Its Effect on Biomass Recalcitrance. Brazilian Journal of Botany 35(4): 303–311.
Costa, F. S. 2018 Análise Mensal Cana-de-açúcar. Conab. (http://www.conab.gov.br). Accessed 12 July 2018.
Creste, S., K.A.G. Accoroni, L.R. Pinto, R. Vencosvskv, M.A. Gimenes, M.A. Xavier, and M.G.A. Landell. 2010. Genetic Variability Among Sugarcane Genotypes Based on Polymorphism in Sucrose Metabolism and Drought Tolerance Genes. Euphytica 172(3): 435–446.
Earl, D., and B. Von-Holdt. 2010. STRUCTURE HARVESTER: a Website and Program for Visualizing STRUCTURE Output and Implementing the EVANNO Method. Conservation Genetic Resources 4(2): 359–361.
Evanno, G., S. Regnaut, and J. Goudet. 2005. Detecting the Number of Clusters of Individuals Using the Software STRUCTURE: A Simulation Study. Molecular Ecology 14: 2611–2620.
Excofier, L., and H. Lischer. 2010. Arlequin Suite ver. 3.5: A New Series of Programs to Perform Population Genetics Analyses Under Linux and Windows. Molecular Ecology Resources 10: 564–567.
Ferrari, F. 2010. Caracterização Cromossômica em Cana-de-Açúcar (Saccharum spp., Poacea). Master’s thesis, University of Campinas, Campinas.
Govindaraj, P., V.A. Amalraj, K. Mohanraj, and N.V. Nair. 2014. Collection, Characterization and Phenotypic Diversity of Saccharum spontaneum L. from Arid and Semi Arid Zones of Northwestern India. Sugar Tech 16(1): 36–43.
Grivet, L., C. Daniels, J.C. Glaszmann, and A. D’hont. 2004. A Review of Recent Molecular Genetics Evidence for Sugarcane Evolution and Domestication. Ethnobotanic Research and Applications 2: 9–17.
Hu, J., and B.A. Vick. 2003. Target Region Amplification Polymorphism: A Novel Marker Technique for Plant Genotyping. Plant Molecular Biology Reporter 21(3): 289–294.
Landell, M.G.A., M.S. Scarpari, M.A. Xavier, I.A. Anjos, A.S. Baptista, C.L. Aguiar, D.N. Silva, M.A.P. Bidóia, S.R. Brancalião, J.A. Bressiani, M.F. Campos, P.E.M. Miguel, T.N. Silva, V.H.P. Silva, L.O.S. Anjos, and B.H. Ogata. 2013. Residual Biomass Potential of Commercial and Pre-commercial Sugarcane Cultivars. Scientia Agricola 70(5): 299–304.
Li, G., and C.F. Quiros. 2001. Sequence-Related Amplified Polymorphism (SRAP), a New Marker System Based on a Simple PCR Reaction: Its Application to Mapping and Gene Tagging in Brassica. Theoretical and Applied Genetics 103: 455–461.
Manechini, J.R.V., J.B. Costa, B.T. Pereira, L.A. Carlini-Garcia, M.A. Xavier, M.G.A. Landell, and L.R. Pinto. 2018. Unraveling the Genetic Structure of Brazilian Commercial Sugarcane Cultivars Through Microsatellite Markers. PLoS ONE 13(4): e0195623. https://doi.org/10.1371/journal.pone.0195623.
Melloni, M.L.G., M.N.G. Melloni, M.S. Maximiliano, J.C. Garcia, M.G.A. Landell, and L.R. Pinto. 2015. Flowering of Sugarcane Genotypes Under Different Artificial Photoperiod Conditions. American Journal of Plant Sciences 6: 456–463.
Melloni, M.N.G., M.L.G. Melloni, A.C. Neuber, D. Perecin, M.G.A. Landell, and L.R. Pinto. 2016. Efficiency of Different Antimitotics in Cytological Preparations of Sugarcane. Sugar Tech 18(2): 222–228.
Nayak, S.N., J. Song, A. Villa, B. Pathak, T. Ayala-Silva, X. Yang, J. Todd, N.C. Glynn, D.N. Kuhn, B. Glaz, R.A. Gilbert, J.C. Comstock, and J. Wang. 2014. Promoting Utilization of Saccharum spp. Genetic Resources Through Genetic Diversity Analysis and Core Collection Construction. PLOS ONE 9(10): e110856. https://doi.org/10.1371/journal.pone.0110856.
Panje, R.R., and C.N. Babu. 1960. Studies in Saccharum spontaneum: Distribution and Geographical Association of Chromosome Numbers. Cytologia 25: 152–172.
Perrier, X., and J. Jacquemoud-Collet. DarWIN software. 2006. 56.
Piperidis, N., G. Piperidis, and A. D’Hont. 2010. Molecular Cytogenetics. In Genetics, Genomics and Breeding of Sugarcane, ed. R. Henry and C. Kole, 9–19. Enfield: Science Publishers.
Portieles, R., R. Rodríguez, and M.T. Cornide. 2004. Cytogenetic Characterization of New Wild Clones of the Saccharum Complex. Cultivos Tropicales 25(1): 17–22.
Pritchard, J., M. Stephens, and P. Donnelly. 2000. Inference of Population Structure Using Multilocus Genotype Data. Genetics 155(2): 945–959.
Ramdoyal, K., and G.H. Badaloo. 2002. Prebreeding in Sugarcane with an Emphasis on the Programme of the Mauritius Sugar Industry Research Institute. https://www.researchgate.net/publication/267260870. Accessed 20 March 2019.
Rezende, C.A., M.A. Lima, P. Maziero, E.R. Azevedo, W. Garcia, and I. Polikarpov. 2011. Chemical and Morphological Characterization of Sugarcane Bagasse Submitted to a Delignification Process for Enhanced Enzymatic Digestibility. Biotechnology for Biofuels 4: 54.
Sarath Padmanabhan, T.S., and G. Hemaprabha. 2018. Genetic Diversity and Population Structure Among 133 Elite Genotypes of Sugarcane (Saccharum spp.) for Use as Parents in Sugarcane Varietal Improvement. 3 Biotech 8: 339. https://doi.org/10.1007/s13205-018-1364-2.
Saitou, N., and M. Nei. 1987. The Neighbor-Joining Method: A New Method for Reconstructing Phylogenetic Trees. Molecular Biology and Evolution 4(4): 406–425.
Shrivastava, A.K., and S. Srivastava. 2016. Diversity of the Germplasm of Saccharum Species and Related Genera Available for use in Directed Breeding Programmes for Sugarcane Improvement. Current Science 111(3): 475–481.
Silva, J.A.G., P.M.A. Costa, T.G. Marconi, E.J.S. Barreto, N. Solís-Gracia, J.-W. Park, and N.C. Glynn. 2018. Agronomic and Molecular Characterization of Wild Germplasm Saccharum spontaneum for Sugarcane and Energy Cane Breeding Purposes. Scientia Agricola 75: 329–338.
Sobhakumari, V.P. 2013. New Determinations of Somatic Chromosome Number in Cultivated and Wild Species of Saccharum. Caryologia: International Journal of Cytology, Cytosystematics and Cytogenetics 66(3): 268–274.
Song, J., X.M.F.R. Yang, L.G. Resende, J. Neves, J. Todd, J.C. Zhang, J. Comstock, and J. Wang. 2016. Natural Allelic Variations in Highly Polyploidy Saccharum Complex. Frontiers in Plant Science 8(7): 804. https://doi.org/10.3389/fpls.2016.00804.
Suman, A., C.A. Kimbeng, S.J. Edme, and J. Veremis. 2008. Sequence-Related Amplified Polymorphism (SRAP) Markers for Assessing Genetic Relationships and Diversity in Sugarcane Germplasm Collections. Plant Genet Resources: Characterization and Utilization 6(3): 222–231.
Suman, A., K. Ali, J. Arro, A.S. Parco, C.A. Kimbeng, and N. Baisakh. 2012. Molecular Diversity Among Members of the Saccharum Complex Assessed Using TRAP Markers Based on Lignin-Related Genes. BioEnergy Research 5(1): 197–205.
Vieira, M.L.C., C.B. Almeida, C.A. Oliveira, L.O. Tacuatiá, C.F. Munhoz, L.A. Cauz-Santos, L.R. Pinto, C.B. Monteiro-Vitorello, M.A. Xavier, and E.R. Forni-Martins. 2018. Revisiting Meiosis in Sugarcane: Chromosomal Irregularities and the Prevalence of Bivalent Configurations. Frontiers in Plant Science 9: 1–12. https://doi.org/10.3389/fgene.2018.00213.
Yang, X., J. Song, J. Todd, Z. Peng, D. Paudel, Z. Luo, X. Ma, Q. You, E. Hanson, Z. Zhao, Y. Zhao, J. Zhang, R. Ming, and J. Wang. 2018. Target Enrichment Sequencing of 307 Germplasm Accessions Identified Ancestry of Ancient and Modern Hybrids and Signatures of Adaptation and Selection in Sugarcane (Saccharum spp.), a ‘sweet’ Crop with ‘bitter’ Genomes. Plant Biotechnology Journal 17(2): 1–11.
Wright, S. 1978. Evolution and Genetics of Populations: Variability Within and Among Natural Populations. Chicago: University of Chicago Press.
Acknowledgements
The authors are grateful to the Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP Grant: 2013/22500-5).
Funding
This study was funded by FAPESP (Fundação de Amparo à Pesquisa do Estado de São Paulo (2013/22500-5).
Author information
Authors and Affiliations
Contributions
CADKJ contributed to data analysis and manuscript writing. JRVM helped in data organization and analysis. JBC and TMF worked for DNA extraction and genotyping. ACRP contributed to manuscript writing and editing. LRP and RXC helped in design and conceptualization of the experiment and research funding. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Junior, C.A.D.K., Manechini, J.R.V., Corrêa, R.X. et al. Genetic Structure Analysis in Sugarcane (Saccharum spp.) Using Target Region Amplification Polymorphism (TRAP) Markers Based on Sugar- and Lignin-Related Genes and Potential Application in Core Collection Development. Sugar Tech 22, 641–654 (2020). https://doi.org/10.1007/s12355-019-00791-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12355-019-00791-0