Abstract
Introduction
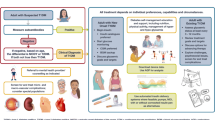
The TOujeo BEyond glucose control (TOBE) study evaluated clinical outcomes with insulin glargine 300 units/mL (Gla-300) in insulin-naïve Korean people with type 2 diabetes mellitus (T2DM) in a real-world setting.
Methods
This 24-week, prospective, non-interventional, multicenter, open-label, single-arm, observational study included adults aged ≥ 20 years with T2DM suboptimally controlled with oral hypoglycemic agents and/or glucagon-like peptide 1 receptor agonists who require basal insulin. Eligible participants were assigned to either general target glycated hemoglobin (HbA1c < 7%) or individualized target groups as per physician’s discretion considering guidelines and participants’ characteristics. The primary endpoint was the proportion of participants achieving the HbA1c target (individualized or general) at 24 weeks.
Results
Among 369 participants, 19.5% (72/369) of participants achieved the HbA1c target at week 24; 37.5% (33/88) in the individualized and 13.9% (39/281) in the general target group. In both target groups, similar reductions in fasting plasma glucose and body weight were observed, with low incidence of hypoglycemia, and T2DM duration was significantly shorter in participants who did versus those who did not achieve the target HbA1c (individualized target group: 9.6 ± 8.0 versus 13.1 ± 8.4 years, P = 0.0454; general target group: 10.2 ± 8.6 versus 12.8 ± 7.4 years, P = 0.0378).
Conclusions
This study showed that initiation of insulin therapy with Gla-300 in people with T2DM using an individualized approach is more effective in achieving an HbA1c target. Moreover, earlier initiation of insulin therapy in people with suboptimally controlled T2DM may increase the success rate of glycemic control.
A graphical abstract is available with this article.
Graphical Abstract

Plain Language Summary
Despite various efforts in managing diabetes, individuals with type 2 diabetes mellitus (T2DM) encounter numerous challenges to achieve good glycemic control. The major cause is failure to initiate insulin therapy in a timely manner, primarily because of the fear of hypoglycemia. Insulin glargine 300 units/mL (Gla-300) has smooth and prolonged activity resulting in stable and sustained glycemic control, thus reducing the risk of hypoglycemia. Studies on efficacy and safety of Gla-300 in various populations have been published globally. However, there are limited real-world studies in Asian populations. This study evaluated effectiveness and safety of Gla-300 in Korean people with T2DM who were not on insulin prior to this study but were taking oral glucose-lowering medications. The participants were assigned to two groups: general glycated hemoglobin (HbA1c) target (HbA1c < 7%) and individualized HbA1c target according to the participant’s characteristics. Results showed that Gla-300 helped to achieve the glycemic target more effectively using an individualized approach. In both groups, similar reductions in fasting plasma glucose and body weight were observed, with low incidence of hypoglycemia. People who achieve glycemic target had a shorter duration of T2DM than those who did not achieve their glycemic target. This suggests that earlier insulin initiation may be a better approach and may increase the success rate of insulin therapy.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
Insulin glargine 300 units/mL (Gla-300) as second-generation basal insulin analogue demonstrated efficacy and safety in randomized controlled trials and real-world studies globally. However, there are limited real-world evidence for Gla-300 in Asian populations. |
The TOujeo BEyond glucose control (TOBE) study gives insights into the effectiveness and safety of Gla-300 in insulin-naïve Korean people with type 2 diabetes mellitus (T2DM) using different glycated hemoglobin (HbA1c) targets in a real-world clinical setting. |
What was learned from the study? |
The proportion of participants who achieved the HbA1c target in the individualized group (37.5%) was almost three times higher than the proportion of participants in the general target group (13.9%). |
Gla-300 is a well-tolerated treatment option for younger and older age groups of insulin-naïve Korean participants with T2DM. |
The duration of T2DM was significantly shorter in participants who achieved their HbA1c target than in those who did not, confirming that earlier insulin initiation may be a better approach to insulin therapy. |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.25273465.
Introduction
Diabetes remains a major global public health issue despite advances in treatment [1,2,3], with an estimated 537 million people worldwide living with diabetes according to 2021 data from the International Diabetes Federation (IDF) [1]. The vast majority of people with diabetes, more than 95%, have type 2 diabetes mellitus (T2DM) [3]. The global prevalence of diabetes in adults has increased steadily over the past few decades. The IDF predicts that 643 million people, or 11.3% of the world population, will have diabetes by 2030 if appropriate action is not taken to address the pandemic [1]. Furthermore, T2DM is the eighth leading cause of mortality and morbidity in terms of disability-adjusted life years (DALYs) [4].
Similar to the rest of the world, T2DM is a significant cause of morbidity and mortality in South Korea [5,6,7], and its prevalence has been increasing [5, 7, 8], particularly in older people [8]. In 2020, an estimated 6.05 million Korean adults aged 30 years or more (16.7% of the population) had T2DM [5]. However, despite the importance of managing diabetes, only 24.5% and 55.6% of individuals with diabetes achieve good glycemic control, defined as a glycated hemoglobin (HbA1c) target < 6.5% and < 7.0%, respectively [5]. A possible reason for the high rate of poor glycemic control is clinical inertia, i.e., the failure to initiate or intensify therapy in a timely manner, which is common in routine clinical practice [9,10,11,12,13]. In order to achieve optimal results, it is important that diabetes management should take an individualized approach to each individual, as emphasized by the American Diabetes Association (ADA) [14] and the Korean Diabetes Association (KDA) guidelines [15]. The KDA recommends that insulin therapy should be initiated if suboptimally controlled with their target glycemic goal despite appropriate treatment with oral hypoglycemic agents (OHAs) or when T2DM is diagnosed in the presence of metabolic decompensation, HbA1c > 9.0%, and/or symptomatic hyperglycemia [15, 16]. The initial choice of insulin treatment should be a basal insulin regimen or premixed insulin injection (once or twice daily) depending on the individuals’ circumstances, and long-acting basal analogues, such as insulin glargine, are preferred to neutral protamine Hagedorn to reduce the risk of hypoglycemia [16].
A second-generation, long-acting basal insulin, insulin glargine 300 units/mL (Gla-300) was developed to mimic the physiologic profile of endogenous basal insulin secretion more accurately. Compared with insulin glargine 100 units/mL (Gla-100), Gla-300 is characterized by a flatter pharmacokinetic and pharmacodynamic profile with a longer duration of action, which results in effective blood glucose control for more than 24 h [17]. The efficacy and safety of Gla-300 have been demonstrated in randomized controlled trials, real-world studies, special populations, and fasting during Ramadan [18,19,20,21,22,23,24,25,26]. However, to date, most clinical trials and real-world studies of Gla-300 have mainly included non-Asians; indeed, fewer studies have included Asians [19, 26]. Thus, the aim of this study was to evaluate the efficacy and safety of Gla-300 in insulin-naïve Korean people with T2DM in a real-world clinical setting.
Methods
Study Design and Population
The TOBE (TOujeo BEyond glucose control) study was a 24-week, prospective, multicenter, open-label, single-arm, non-interventional study to assess the efficacy and safety of Gla-300 (Toujeo®, Sanofi) in routine clinical practice in insulin-naïve Korean people with T2DM and inadequate glycemic control. Eligible participants were male and female adults aged ≥ 20 years with T2DM who were not achieving an individual glucose target (based on HbA1c) despite being prescribed at least one OHA and/or a glucagon-like peptide 1 receptor agonist (GLP-1 RA) for 3 months or more and who required basal insulin at the time of enrollment according to the judgment of the investigator. Participants with T2DM were enrolled from 27 representative endocrinology centers in South Korea from June 2016 to July 2019. Exclusion criteria included participants with type 1 diabetes, participants with T2DM who required short-acting insulin or basal insulin in combination with short-acting insulin, use of any product containing insulin since the diagnosis of T2DM, and the use of any investigational agent within 3 months prior to enrollment.
All study participants were assigned to either the individualized target group, with an individualized target set according to the HbA1c level that the attending physician aimed to achieve by 24 weeks, or the general target group (HbA1c < 7.0%), with no individualized target set. The individualized HbA1c targets were determined according to the current ADA [14] or KDA [15] guidelines, taking into consideration the clinical features such as age, comorbidities, hypoglycemia risk, and cardiovascular risk. The initial dose was based on the label recommendation and titration of Gla-300 was determined by the investigator according to fasting plasma glucose (FPG) and HbA1c targets. The OHA and/or GLP-1 RA prescribed prior to study entry could either be maintained or changed at the investigator’s discretion.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review board at each of the 27 study sites (see Supplementary Materials Table S1), starting from first approval at Kyung Hee University Hospital at Gangdong Institutional Review Board (Approval code KHNMC 2016-05-005, Approval date 2016-05-20). All participants provided written, informed consent prior to enrollment and for anonymized patient information to be published in this article.
Study Endpoints
The primary endpoint was the proportion of participants achieving their HbA1c target at 24 weeks. Secondary efficacy endpoints were the change from baseline to week 24 in HbA1c, FPG, and body weight, the proportion of participants with an individualized or general HbA1c target who achieved the target at any time once reached the target during the study, the proportion of participants who achieved their individualized HbA1c target at week 24 without documented symptomatic hypoglycemia, participants’ persistence with Gla-300, and changes in Gla-300 dose from study initiation to week 24. Secondary safety endpoints included the incidence and event rate of documented symptomatic nocturnal and 24-h hypoglycemia (blood glucose level ≤ 70 mg/dL) and severe hypoglycemia (< 54 mg/dL) during the 24-week study period. Additional endpoints were the duration of exposure to Gla-300 and the occurrence of treatment-emergent adverse events (TEAEs), defined as adverse events (AEs) occurring during the study period. The event rate was calculated as the incidence of hypoglycemia/total person-years of participants included in the safety analysis set, where total person-years was equal to the sum of the date of the last dose minus the date of the first dose 1/365.25 for all evaluable participants in the analysis population.
Statistical Analysis
Sample size was calculated on the basis of the primary endpoint (proportion of participants achieving their individual HbA1c goals in the actual clinical setting). Assuming a projected rate of 40% of participants reaching the HbA1c target and a bilateral significance level of 0.05, a minimum of 369 participants would meet the maximum tolerance of 5% in estimating the target achievement rate [18, 27]. Considering the proportion of participants who could not be evaluated as 30%, the total number of subjects was calculated to be 527, which justifies the study sample size.
Descriptive statistics were used for continuous demographic data, with frequency and percentage used for categorical data. Continuous variables are presented as mean ± standard deviation. The proportion and 95% confidence intervals (CIs) were used for the primary endpoint. The analysis of secondary endpoints included descriptive statistics, 95% CIs, and paired t test or Wilcoxon’s signed rank test, as appropriate. The incidence, event rate, and 95% CIs were used for documented symptomatic hypoglycemia, severe hypoglycemia, and nocturnal hypoglycemia.
The efficacy population analyzed was the full analysis set (FAS), defined as participants who received at least one dose of Gla-300 and had an HbA1c measurement at week 24. Safety was assessed in the safety analysis set, defined as participants who received at least one dose of Gla-300 during the study period.
Results
Study Population and Baseline Characteristics
A total of 531 participants were screened at 27 university and general hospitals in South Korea; there were 36 screening failures, and one participant was excluded because of the loss of the informed consent form, resulting in 494 participants being enrolled (Supplementary Materials Fig. S1). Of the 494 enrolled participants, 406 completed the study and 369 were included in the FAS. In the FAS, 88 participants (23.8%) were included in the individualized target group and 281 participants (76.2%) were included in the general target group. In the individualized HbA1c target group, the HbA1c targets determined by the investigators were < 6% (2.3% of participants), < 6.5% (20.5%), < 7% (55.7%), < 7.5% (6.8%), < 8% (10.2%), < 8.5% (3.4%), and < 9% (1.1%). The safety analysis set included 488 participants who received at least one dose of Gla-300.
Baseline demographics and clinical characteristics of participants in the FAS and those who did or did not achieve their individualized or general HbA1c target are shown in Table 1 and Supplementary Materials Table S2. Overall, the mean age was 60.2 ± 11.4 years, mean duration of T2DM was 12.2 ± 7.8 years, and 207 participants (56.1%) were male. The mean HbA1c was 9.9 ± 1.6% and mean FPG was 224.3 ± 76.9 mg/dL. In total, 127 participants (34.4%) had one or more diabetes complication, the most common of which were diabetic neuropathy (20.3%, 75/369), diabetic retinopathy (11.4%, 42/369), and diabetic nephropathy (4.3%, 16/369). Most participants (93.5%, 345/369) had at least one comorbidity, which included dyslipidemia, hypertension, arteriosclerosis, chronic gastritis, and chronic kidney disease.
Most of the baseline demographics and clinical characteristics were generally similar between participants who did and did not achieve their HbA1c target in each study group. However, those participants achieving their glycemic target in each study group had a significantly shorter duration of T2DM than those who did not (individualized target group: 9.6 ± 8.0 versus 13.1 ± 8.4 years, P = 0.0454; general target group: 10.2 ± 8.6 versus 12.8 ± 7.4 years, P = 0.0378). Fewer participants in the individualized target group had diabetic complications compared with the general target group (18 versus 109, P = 0.0016).
Concomitant Medications
Before study enrollment, all participants received diabetic medications, including sulfonylureas (25.9%), dipeptidyl peptidase 4 (DPP4) inhibitors (24.6%), metformin (22.2%), and a fixed-dose combination of metformin and a DPP4 inhibitor (10.5%). At the end of study, the most used diabetic medication was metformin (24.0%), followed by DPP4 inhibitors (13.5%), a fixed-dose combination of metformin and a DPP4 inhibitor (13.5%), and sulfonylureas (9.0%). There were no significant differences between the individualized and general target groups (Supplementary Materials Table S3). Most participants (91.1%, 336/369) were also taking concomitant medications before the trial including lipid-lowering agents (73.0%, 269/369) and antithrombotic agents (46.6%, 172/369).
Efficacy Endpoints
A total of 72 participants (19.5%) achieved their HbA1c target at 24 week; 33 of 88 (37.5%) participants in the individualized target group and 39 of 281 participants (13.9%) in the general target group (HbA1c < 7%), (P < 0.0001; Table 2). Even if the HbA1c target < 7% was applied to both groups, significant differences in the proportions remained: 23 of 88 (26.1%) participants in the individualized target group versus 39 of 281 (13.9%) participants of the general target group (P < 0.0073). The rates of HbA1c target achievement in the individualized target group were as follows: < 6% (50.0%), < 6.5% (38.9%), < 7% (26.5%), < 7.5% (33.3%), < 8% (66.7%), < 8.5% (100%), and < 9% (100%). The mean HbA1c level in the overall study population at week 24 was 8.4% ± 1.6% and the mean change from baseline to week 24 was − 1.5% ± 1.9% (week 24 vs. baseline: P < 0.0001) (Table 2). The proportion of participants who achieved their glycemic target at any time during the study in the individualized target group, general target group, and the overall population was 43.2% (38/88 participants), 17.1% (48/281 participants; P < 0.0001), and 23.3% (86/369 participants), respectively (Fig. 1a). The proportion of participants who achieved their individualized HbA1c target at week 24 without documented symptomatic hypoglycemia during the study was 45.3% (34/75 participants).
At week 24, the mean FPG level in the overall study population was 157.6 ± 58.7 mg/dL and the mean change from baseline was − 68.7 ± 80.5 mg/dL (P < 0.0001) (Table 2). The mean body weight in the overall population at week 24 was 66.5 ± 10.9 kg and the mean change from baseline to week 24 was 0.9 ± 4.1 kg (P = 0.0016) (Table 2).
The mean Gla-300 dose was 17.5 ± 8.5 U at week 24, a mean increase of 4.1 ± 6.7 U (P < 0.0001), and there was no significant difference between the two groups (Table 2). During the 24-week study period, 84.3% of participants persisted with Gla-300 treatment. The mean duration of treatment with Gla-300 was 159.4 ± 68.2 days. The mean daily and total Gla-300 dose was 16.2 ± 7.1 U and 2613.1 ± 2044.5 U per participant, respectively.
Safety
TEAEs in the safety analysis set (N = 488) are shown in Table 3. In total, 132 participants (27.1%; 95% CI 23.1, 31.0) reported 210 TEAEs, the majority of which were mild (157 events) or moderate (46 events) and only seven events were severe. A causal relationship with Gla-300 was defined as certain for two events, probable/likely for one event, possible for 11 events, not likely/unlikely for 190 events, and not assessable/classifiable for six events. The most common TEAEs were hyperglycemia (2.0%, n = 10; 10 events), diarrhea (1.8%, n = 9; 10 events), dizziness (1.6%, n = 8; eight events), and nasopharyngitis (1.4%, n = 7; eight events). 38 participants (7.8%; 95% CI 5.41, 10.16; 39 events) reported serious TEAEs. The most common serious TEAE was uncontrolled hyperglycemia.
TEAEs leading to the temporary interruption of Gla-300 occurred in four participants (0.8%; 95% CI 0.22, 2.09; four events): hospitalization for hyperglycemia in two participants (0.4%; two events), and abdominal discomfort and renal cell carcinoma each in one participant (0.2%; one event). TEAEs leading to the permanent discontinuation of Gla-300 occurred in 15 participants (3.1%; 95% CI 1.54, 4.61; 16 events). The most common TEAEs leading to permanent discontinuation of Gla-300 were hospitalization for hyperglycemia in three participants (0.6%; three events), and edema in two participants (0.4%, two events). Three deaths occurred during the study, one due to hepatocellular carcinoma, one due to malignant neoplasm of the ampulla of Vater, and one with no recorded cause.
The incidence and event rate of documented symptomatic 24-h hypoglycemia was 9.0% (n = 44, 63 events) and 0.30 events per participant-year, respectively, in the overall population. The rate of severe documented hypoglycemia was 0.2% (n = 1, one event). The incidence and event rate of nocturnal hypoglycemia was 1.0% (n = 5, seven events) and 0.03 events per participant-year, respectively. There were no cases of severe nocturnal hypoglycemia. In the FAS, the incidence of hypoglycemia was 14.8% (13/88 participants), 8.9% (25/281 participants), and 10.3% (38/369 participants) in the individualized target group, general target group, and overall population, respectively (Fig. 1b).
Efficacy and Hypoglycemia Outcomes in Participants Aged ≥ 65 vs. < 65 Years
In participants aged ≥ 65 years, the mean HbA1c at baseline was 9.7% ± 1.5%, the mean change at week 24 was − 1.4 ± 1.8% (P < 0.0001), and the proportion of participants achieving an individualized or general HbA1c target at week 24 was 18.8% (26/138) (Table 4). In participants aged < 65 years, the mean HbA1c at baseline was 10.0% ± 1.7%, the mean change at week 24 was − 1.6 ± 2.0% (P < 0.0001), and the proportion of participants achieving their HbA1c target at week 24 was 19.9% (46/231). The incidence of hypoglycemia was 6.5% (n = 9) in participants aged ≥ 65 years and 12.6% (n = 29) in those aged < 65 years (P = 0.0651).
Discussion
Individualized treatment goals for T2DM are already very common in routine clinical practice. Many guidelines recommend individualized treatment, and particularly, in the real world, the recommendation is to establish an optimized treatment strategy centered on the person [14]. In our study, the proportion of participants who achieved the HbA1c target in the individualized group (37.5%) was almost three times higher than the proportion of participants in the general target group (13.9%). This is a higher proportion than 25.2% of participants who achieved their individualized glycemic target at month 6 (the primary endpoint) in ATOS (A TOUJEO Observational Study), which was conducted in insulin-naïve participants in a real-world setting in Asia, the Middle East, North Africa, Latin America, and Eastern Europe [26]. However, in ATOS, all participants had a pre-defined individualized HbA1c target determined by their treating physician.
The importance of early intensive glycemic control has been demonstrated in randomized clinical trials [28, 29]. In a post hoc pooled analysis of 16 randomized, treat-to-target clinical trials of Gla-100, a shorter duration of T2DM was one of the factors found to be associated with a good glycemic response [30]. In our study, in both the individualized and general target groups, the duration of T2DM in participants who achieved their HbA1c target was significantly shorter than in those who did not. The results indicate that participants are more likely achieve their HbA1c target with earlier initiation of insulin treatment, confirming the importance of this treatment strategy. Despite the evidence for the benefits of early intensive glucose control in South Korea [28], the initiation of insulin treatment continues to be delayed. In the Modality of Insulin Treatment Evaluation (MOTIV) study, a real-world study investigating insulin initiation in 8636 insulin-naïve Korean people with T2DM and inadequate glycemic control with OHAs from year 2007 to 2009, the majority of whom (> 99%) received Gla-100, mean HbA1c prior to insulin initiation was 9.2% and the mean duration of T2DM was 8.9 years [27]. In this study, 44.5% of participants achieved the HbA1c target of < 7.0% at month 6 [27]. In our study, at baseline, the mean HbA1c was 9.9%, and the mean duration of T2DM was 12.2 years, both of which are higher than in the MOTIV study, which may account for the slightly smaller proportion of participants in our study achieving their HbA1c target.
Our TOBE study is the first observational real-world study of Gla-300 in the treatment of Korean people with T2DM using different HbA1c targets. Studies of Gla-300 conducted to date have mainly included non-Asian populations, except for the EDITION JP2 study, which was conducted in insulin-experienced Japanese participants [31, 32] and the EDITION AP study, which was conducted in insulin-naïve participants in China, South Korea, and Taiwan [19]. In the EDITION AP randomized controlled trial, in which participants had a mean duration of T2DM of 10.6 years and a mean HbA1c of 8.6% at baseline, 19.1% of participants achieved the HbA1c target, with no hypoglycemia, during the last 12 weeks of treatment [19]. Although comparisons are difficult because of differences in study design and participant populations, our study showed a much higher proportion of participants (45.3%) achieving an individualized HbA1c target without hypoglycemia than in the EDITION trial despite a longer duration of T2DM (12.2 years) and a higher mean HbA1c at baseline (9.9%) [19].
In our study, Gla-300 was also associated with a low incidence and event rate of documented symptomatic 24-h hypoglycemia, 9.0% and 0.30 events per participant-year, respectively, and there were no cases of documented symptomatic severe hypoglycemia. Compared with the MOTIV study of Gla-100, the incidence of hypoglycemia in this TOBE study was substantially lower (17.6% vs. 10.3%) [27]. There was a significant increase in mean Gla-300 dose of 4.0 U from study initiation to week 24 (P < 0.0001). This compares with a significant increase of 7.6 IU in the Gla-100 dose from initiation to month 6 in the MOTIV study (P < 0.001) [27]. On the basis of the results of this real-world study, active insulin dose titration in the routine clinical setting is important for participants to achieve their HbA1c target. Furthermore, Gla-300 was well tolerated in this TOBE study. The majority of TEAEs were mild or moderate in severity and the incidence was low, with the most common TEAEs being experienced by less than 2% of participants. Although three deaths occurred during the study, these were attributable to underlying medical conditions and were not related to Gla-300 treatment.
Glycemic targets must be individualized in the context of shared decision-making to address the needs and preferences of each person and the individual characteristics that influence the risks and benefits of therapy for each person to optimize their engagement and self-efficacy [14]. Guidelines suggest setting an individualized glycemic goal according to the duration of diabetes, life expectancy, comorbid conditions, complications, and the individual’s preference [14, 15]. Older people are at high risk of hypoglycemia, so an individualized approach is recommended [14]. Furthermore, older people with diabetes may be at greater risk of hypoglycemic unawareness and hypoglycemia may be more serious in older than in younger people. In addition, T2DM may be more difficult to control in the elderly because of the increased likelihood of comorbidities and the use of concomitant medications. In our study, 37% of the participants were aged ≥ 65 years and the efficacy of Gla-300 in older (≥ 65 years) versus younger (< 65 years) participants was compared. There was no significant difference in the proportion of older and younger participants achieving their HbA1c target goal at week 24, the mean change from baseline in HbA1c at week 24, and the incidence of hypoglycemia, indicating that Gla-300 is an effective and well-tolerated treatment option for older participants.
However, it is important to acknowledge the limitations of this study. First, as a result of the inherent nature of this being an open-label, observational study with a lack of comparator arm, there could be potential observer and selection bias. Second, since Gla-300 was co-administered with different OADs, we could not evaluate the effects of these OADs on the achievement of blood glucose targets. The use of different OADs could have been a confounder that might have influenced the efficacy of Gla-300. Third, the high dropouts rate of 25% resulted in 369 in FAS group out of 531 participants. However, considering the nature of the study, certain dropouts and missing data were expected and the sample size was calculated accordingly. Therefore, regardless of ths high dropout rate, available data were sufficient to evaluate efficacy of Gla-300.
Conclusion
This prospective observational study shows that both earlier initiation of insulin therapy and treatment of T2DM according to individualized goals are important factors in achieving an HbA1c target and that Gla-300 is an effective and well-tolerated insulin treatment option.
Data Availability
All data generated or analyzed during this study are included in this published article/as supplementary information files. Qualified researchers may request access to participant-level data and related documents [including, e.g., the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications]. Participant-level data will be anonymized, and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi's data sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
References
International Diabetes Federation (IDF). IDF diabetes atlas. 10th edition 2021. https://www.diabetesatlas.org/en/. Accessed Feb 13, 2024.
World Health Organization (WHO). Global report on diabetes. 2016. https://www.who.int/diabetes/global-report/en/. Accessed Feb 13, 2024.
World Health Organization (WHO). Diabetes fact sheet. 5 April 2023. https://www.who.int/news-room/fact-sheets/detail/diabetes. Accessed Feb 13, 2024.
GBD 2021 Diabetes Collaborators. Global, regional, and national burden of diabetes from 1990 to 2021, with projections of prevalence to 2050: a systematic analysis for the Global Burden of Disease Study 2021. Lancet. 2023;2023(402):203–34.
Bae JH, Han KD, Ko SH, et al. Diabetes fact sheet in Korea, 2021. Diabetes Metab J. 2022;46:417–26.
Ko SH, Han K, Lee YH, et al. Past and current status of adult type 2 diabetes mellitus management in Korea: a national health insurance service database analysis. Diabetes Metab J. 2018;42:93–100.
Noh J. The diabetes epidemic in Korea. Endocrinol Metab (Seoul). 2016;31:349–53.
Koo BK, Moon MK. Are we in the same risk of diabetes mellitus? Gender- and age-specific epidemiology of diabetes in 2001 to 2014 in the Korean population. Diabetes Metab J. 2016;40:175–81.
Ali SN, Dang-Tan T, Valentine WJ, Hansen BB. Evaluation of the clinical and economic burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the United States. Adv Ther. 2020;37:869–82.
Bain SC, Bekker Hansen B, Hunt B, Chubb B, Valentine WJ. Evaluating the burden of poor glycemic control associated with therapeutic inertia in patients with type 2 diabetes in the UK. J Med Econ. 2020;23:98–105.
Goh SY, Ang E, Bajpai S, et al. A patient-centric approach to optimise insulin therapy in Asia. J Diabetes Complications. 2016;30:973–80.
Khunti S, Khunti K, Seidu S. Therapeutic inertia in type 2 diabetes: prevalence, causes, consequences and methods to overcome inertia. Ther Adv Endocrinol Metab. 2019;10:2042018819844694.
Maegawa H, Ishigaki Y, Langer J, Saotome-Nakamura A, Andersen M, Japan Diabetes Clinical Data Management (JDDM) Study Group. Clinical inertia in patients with type 2 diabetes treated with oral antidiabetic drugs: results from a Japanese cohort study (JDDM53). J Diabetes Investig. 2021;12:374–81.
American Diabetes Association. Standards of medical care in diabetes—2021. Diabetes Care. 2021;44(Suppl 1):S1–2.
Kim MK, Ko SH, Kim BY, et al. 2019 Clinical practice guidelines for type 2 diabetes mellitus in Korea. Diabetes Metab J. 2019;43:398–406.
Lee BW, Kim JH, Ko SH, et al. Insulin therapy for adult patients with type 2 diabetes mellitus: a position statement of the Korean Diabetes Association, 2017. Diabetes Metab J. 2017;41:367–73.
Becker RH, Dahmen R, Bergmann K, Lehmann A, Jax T, Heise T. New insulin glargine 300 Units·mL–1 provides a more even activity profile and prolonged glycemic control at steady state compared with insulin glargine 100 Units·mL–1. Diabetes Care. 2015;38:637–43.
Bolli GB, Riddle MC, Bergenstal RM, et al. Glycaemic control and hypoglycaemia with insulin glargine 300U/mL versus insulin glargine 100U/mL in insulin-naïve people with type 2 diabetes: 12-month results from the EDITION 3 trial. Diabetes Metab. 2017;43:351–8.
Ji L, Kang ES, Dong X, et al. Efficacy and safety of insulin glargine 300 U/mL versus insulin glargine 100 U/mL in Asia Pacific insulin-naïve people with type 2 diabetes: the EDITION AP randomized controlled trial. Diabetes Obes Metab. 2020;22:612–21.
Ritzel R, Roussel R, Giaccari A, Vora J, Brulle-Wohlhueter C, Yki-Järvinen H. Better glycaemic control and less hypoglycaemia with insulin glargine 300 U/mL vs glargine 100 U/mL: 1-year patient-level meta-analysis of the EDITION clinical studies in people with type 2 diabetes. Diabetes Obes Metab. 2018;20:541–8.
Yki-Järvinen H, Bergenstal RM, Bolli GB, et al. Glycaemic control and hypoglycaemia with new insulin glargine 300 U/ml versus insulin glargine 100 U/ml in people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: the EDITION 2 randomized 12-month trial including 6-month extension. Diabetes Obes Metab. 2015;17:1142–9.
Bailey TS, Wu J, Zhou FL, et al. Switching to insulin glargine 300 units/mL in real-world older patients with type 2 diabetes (DELIVER 3). Diabetes Obes Metab. 2019;21:2384–93.
Ghosh S, Kalra S, Bantwal G, et al. Use of second-generation basal insulin Gla-300 in special populations: a narrative mini-review. Curr Diabetes Rev. 2023;19:86–95.
Hassanein M, Buyukbese MA, Malek R, et al. Real-world safety and effectiveness of insulin glargine 300 U/mL in participants with type 2 diabetes who fast during Ramadan: the observational ORION study. Diabetes Res Clin Pract. 2020;166:108189.
Bailey TS, Zhou FL, Gupta RA, et al. Glycaemic goal attainment and hypoglycaemia outcomes in type 2 diabetes patients initiating insulin glargine 300 units/mL or 100 units/mL: real-world results from the DELIVER Naïve cohort study. Diabetes Obes Metab. 2019;21:1596–605.
Galstyan GR, Tirosh A, Vargas-Uricoechea H, et al. Real-world effectiveness and safety of insulin glargine 300 u/ml in insulin-naïve people with type 2 diabetes: the ATOS study. Diabetes Ther. 2022;13:1187–202.
Kim SS, Kim IJ, Kim YK, et al. Insulin initiation in insulin-naïve Korean type 2 diabetic patients inadequately controlled on oral antidiabetic drugs in real-world practice: the Modality of Insulin Treatment Evaluation Study. Diabetes Metab J. 2015;39:481–8.
Chon S, Rhee SY, Ahn KJ, et al. Long-term effects on glycaemic control and β-cell preservation of early intensive treatment in patients with newly diagnosed type 2 diabetes: a multicentre randomized trial. Diabetes Obes Metab. 2018;20:1121–30.
UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). UK Prospective Diabetes Study (UKPDS) Group. Lancet. 1998;352:837–53.
Owens DR, Landgraf W, Frier BM, et al. Commencing insulin glargine 100 U/mL therapy in individuals with type 2 diabetes: determinants of achievement of HbA1c goal less than 7.0. Diabetes Obes Metab. 2019;21:321–9.
Terauchi Y, Koyama M, Cheng X, et al. Glycaemic control and hypoglycaemia with insulin glargine 300 U/mL compared with glargine 100 U/mL in Japanese adults with type 2 diabetes using basal insulin plus oral anti-hyperglycaemic drugs (EDITION JP 2 randomised 12-month trial including 6-month extension). Diabetes Metab. 2017;43:446–52.
Terauchi Y, Koyama M, Cheng X, et al. New insulin glargine 300 U/ml versus glargine 100 U/ml in Japanese people with type 2 diabetes using basal insulin and oral antihyperglycaemic drugs: glucose control and hypoglycaemia in a randomized controlled trial (EDITION JP 2). Diabetes Obes Metab. 2016;18:366–74.
Acknowledgements
The authors would like to thank all investigators and participants who participated in the TOBE study.
Medical Writing/Editorial Assistance.
Writing and editorial assistance was provided by Lee Miller of Miller Medical Communications Ltd. (funded by Sanofi Korea) and Daisy Masih, Ph.D. (employee of Sanofi).
Funding
Open Access funding enabled and organized by Seoul National University. This study, the rapid service fee, and the open access fee was sponsored by Sanofi Korea.
Author information
Authors and Affiliations
Contributions
Design: Eun-Gyoung Hong, Hak Chul Jang; Conduct/data collection: Eun-Gyoung Hong, Kyung-Wan Min, Jung Soo Lim, Kyu-Jeung Ahn, Chul Woo Ahn, Jae-Myung Yu, Hye Soon Kim, Hyun Jin Kim, Hak Chul Jang; Analysis: Eun-Gyoung Hong, Kyung-Wan Min, Jung Soo Lim, Kyu-Jeung Ahn, Chul Woo Ahn, Jae-Myung Yu, Hye Soon Kim, Hyun Jin Kim, Hak Chul Jang; Writing manuscript: Eun-Gyoung Hong, Won Kim, Dong Han Kim, Hak Chul Jang; All authors reviewed and approved the final manuscript for publication.
Corresponding author
Ethics declarations
Conflict of Interest
Won Kim and Dong Han Kim are employees of Sanofi Korea. Eun-Gyoung Hong, Kyung-Wan Min, Jung Soo Lim, Kyu-Jeung Ahn, Chul Woo Ahn, Hye Soon Kim, Hyun Jin Kim, and Hak Chul Jang declare no competing interests.
Ethical Approval
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines and was approved by the institutional review board at each of the 27 study sites (see Supplementary Materials Table S1), starting from first approval at Kyung Hee University Hospital at Gangdong Institutional Review Board (Approval code KHNMC 2016-05-005, Approval date 2016–05-20). All participants provided written, informed consent prior to enrollment and for anonymized patient information to be published in this article.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Hong, EG., Min, KW., Lim, J.S. et al. Real-World Outcomes of Individualized Targeted Therapy with Insulin Glargine 300 Units/mL in Insulin-Naïve Korean People with Type 2 Diabetes: TOBE Study. Adv Ther 41, 1967–1982 (2024). https://doi.org/10.1007/s12325-024-02830-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-024-02830-z