Abstract
Cardiac arrest (CA) is a critical public health issue affecting more than half a million Americans annually. The main determinant of outcome post-CA is hypoxic–ischemic brain injury (HIBI), and temperature control is currently the only evidence-based, guideline-recommended intervention targeting secondary brain injury. Temperature control is a key component of a post-CA care bundle; however, conflicting evidence challenges its wide implementation across the vastly heterogeneous population of CA survivors. Here, we critically appraise the available literature on temperature control in HIBI, detail how the evidence has been integrated into clinical practice, and highlight the complications associated with its use and the timing of neuroprognostication after CA. Future clinical trials evaluating different temperature targets, rates of rewarming, duration of cooling, and identifying which patient phenotype benefits from different temperature control methods are needed to address these prevailing knowledge gaps.
Similar content being viewed by others
Hypoxic–ischemic brain injury resulting from cardiac arrest comprise primary and secondary brain injuries with multifactorial mechanisms leading to both types of injury. |
Temperature control remains a cornerstone of post-cardiac arrest care as mechanistic pathways for secondary brain injury are temperature dependent. However, several aspects of this therapy, including target temperature, duration, and methods and rate of rewarming are prevailing knowledge gaps currently being investigated. |
Physiologic complications of hypothermia can affect nearly every organ system, each of which exhibit a temperature-dependent response. |
Pharmacologic management in the post-cardiac arrest patient includes analgosedation, hemodynamic support, prevention and suppression of shivering, and management of the metabolic aberrations during temperature control. |
Neuroprognostication post-CA should be multimodal and delayed for at least 72 h after return of spontaneous circulation, or longer if temperature control was employed, in order to avoid inappropriate withdrawal of life-sustaining therapy. |
Introduction
Out-of-hospital cardiac arrest (OHCA) affects over 600,000 individuals each year in the United States and Europe, while in-hospital cardiac arrest (IHCA) affects ~ 300,000 people a year [1], posing a major public health challenge. Mortality remains unacceptably high, with roughly one in ten OHCA and one in four IHCA patients surviving to hospital discharge [2,3,4,5]. Hypoxic–ischemic brain injury (HIBI) is a major contributor to outcomes after cardiac arrest (CA) [6]; the burden of HIBI is determined by both the primary ischemic injury and the secondary brain injury after reperfusion [7, 8]. To date, there is only one intervention recommended by the American Heart Association and the European Resuscitation Council (ERC) intended to improve neurological outcomes by targeting HIBI: temperature control [9, 10]. Temperature control involves actively modulating core body temperature to a range of 32–37.5 °C for at least 24 h after CA. It is worth noting that “therapeutic hypothermia”, “cooling” and “targeted temperature management” have been used in the past to reflect the practice of controlling temperature, passively or actively, within a certain range, most often between 32 and 36 °C; these are falling out of favor given the changing landscape in post-CA care, where target temperatures may not be in the hypothermic range and the term “targeted temperature management” may suggest practices following the study protocol of specific clinical trials. The premise of temperature control is to mitigate HIBI by minimizing mitochondrial injury, cerebral metabolism, formation of free radicals, and neuronal excitotoxicity pathways that are temperature dependent [7, 11,12,13]. In this scoping review, we characterize the mechanisms implicated in HIBI and dissect post-CA care, with a focus on temperature control and its phases, as well as the implications for neurologic outcome prediction.
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Methods
A literature search in the PubMed database was performed for research articles published up to November 2022, and included any of the terms, Cardiac Arrest, Targeted Temperature Management, Post-Cardiac Arrest Syndrome, Heart Arrest, Temperature Control, and Hypothermia, were reviewed and included in this scoping review. The citations included were analyzed to determine if any additional articles met the inclusion criteria. Only peer-reviewed articles published in the English language were considered for this scoping review.
Mechanisms of Neurologic Injury in Cardiac Arrest
HIBI is one of the most feared complications from CA [14], and encompasses both primary and secondary neuronal injuries. Along with shock-like endotheliopathy, secondary brain injury mechanisms are the cornerstone of the post-resuscitation syndrome, making them appealing targets for interventions aimed at improving neurological outcomes and survival post-CA.
Primary Brain Injury
Primary brain injury occurs during the “no-flow” phase of CA when oxygenation to vital organs becomes compromised. Previous data have reported that electroencephalographic (EEG) attenuation or suppression ensues within 30 s of circulatory arrest. However, in rare cases, EEG activity can continue for several minutes after blood flow ceases [15]. Neuronal death may occur within minutes [16]. This ischemic injury continues until partial reperfusion is restored during cardiopulmonary resuscitation (CPR), and is characterized by depletion of ATP causing dysfunction of ion exchange pumps [6]. The loss of transmembrane ionic gradient leads to anoxic depolarization, a type of spreading depolarization characterized by a massive wave of depolarization, causing cytotoxic edema, release of glutamate, and excitotoxicity. Anoxic depolarization leads to cell death unless bioenergetic supply is restored [17, 18]. Additional mechanisms in primary brain injury include intracellular acidosis, neuronal excitotoxicity, and apoptosis [16]. It is imperative to restore perfusion promptly, as the brain is uniquely intolerant to ischemia, and animal studies have shown that signs of cerebral edema begin to appear on imaging as early as during the initial “no-flow” phase of CA [19].
Secondary Brain Injury
When CPR is initiated and a temporary “low-flow” state ensues, cerebral blood flow (CBF) is restored at ~ 25% of its normal rate [6]. This suboptimal CBF is insufficient to effectively perfuse the brain, and subsequent neuronal damage through complex mechanisms follows. Immediately after the return of spontaneous circulation (ROSC), there is a transient state of “no-reflow”, which is characterized by microcirculatory failure despite restoration of forward flow [20]. Recently, this “no-reflow” phenomenon has been observed in vivo, and is hypothesized to be caused by cortical hypoperfusion due to reduced capillary red blood cell velocity. This multifaceted phenomenon occurs in multifocal areas in the brain parenchyma, and its effect compound on impaired cerebral autoregulation, blood–brain barrier dysfunction, cerebral edema, intracellular Ca2+ accumulation, and release of reactive oxygen and nitrogen species, a cascade which results in further cell death [6, 16, 21, 22]. These physiological changes may continue over the course of days post-arrest, leading to secondary brain injury, and have become the main targets of interventions aiming to mitigate HIBI.
The Role of Temperature Control in Post-Cardiac Arrest Care
Hyperthermia following CA is common, with over 40% CA survivors experiencing elevated core temperatures within 48 h of ROSC. Post-CA hyperthermia has been shown to have deleterious effects on survival and neurological outcomes [13, 23,24,25]. A temperature > 37.5 °C following resuscitation will potentiate neuronal injury by further disrupting the blood–brain barrier, increasing cerebral metabolism, intracellular Ca2+ influx, and enhancing neuronal excitotoxicity, ultimately enhancing cell-death pathways [16]. In addition, hyperthermia post-CA may further impair cerebral autoregulation [26]. For this reason, guidelines universally recommend avoiding hyperthermia (> 37.7 °C) for at least 72 h after ROSC [9, 10].
Temperature control is a standard practice in post-CA care, and involves actively maintaining a temperature between 32 and 37.5 °C for at least 24 h in patients who remain unresponsive to verbal commands after ROSC [9, 27]. Currently, guidelines recommend initiating temperature control as early as possible following ROSC in patients who do not respond to verbal commands, regardless of location of arrest and type of non-perfusing rhythm.
The Scientific Premise of Hypothermia and Neuroprotection
The neuroprotective effects of modulating temperature following HIBI are well established in the literature, and include decreasing cerebral metabolic rate (cerebral metabolic rate decreases by ~ 6% per every 1 °C the temperature is decreased), inhibition of the inflammatory cascade, minimizing release of excitatory neurotransmitters, decreasing neuronal apoptosis, and preserving the blood–brain barrier integrity [9, 28, 29]. This neuroprotection was first demonstrated in humans in a case series of four adult and pediatric patients who experienced CA and were cooled to 30–33 °C via a water-cooled mattress for at least 24 h [30]. At discharge, three patients had complete neurological recovery, while one had severe visual deficits that fully resolved after 1 month. These findings were later confirmed by a slightly larger case series comprising 19 patients who suffered CA; 7 patients did not undergo hypothermia and the remaining 12 were cooled to 31–32 °C with a cooling blanket. Of the 12 patients who were cooled, 50% survived compared to the 14% who survived in the non-treatment group [31]. Although these initial studies were not randomized, controlled trials (RCT), the results were promising; however, research on hypothermia post-CA was almost halted due to difficulty managing complications such as arrhythmias and shivering [32]. In the decades that followed, preclinical studies consistently showed improved neurological outcomes post-CA when hypothermia was implemented during, or shortly after, circulatory arrest [32, 33]. This body of evidence prompted the design of the two landmark trials published in 2002 showing the efficacy of hypothermic temperature control on improving neurological outcomes in OHCA survivors with shockable rhythm who were unresponsive to verbal commands.
Early Clinical Trials on Hypothermia
The Hypothermia After Cardiac Arrest (HACA) study enrolled 275 adults who suffered OHCA with ventricular fibrillation (VF) or pulseless ventricular tachycardia from cardiac etiology, and remained unable to follow commands after ROSC; 55% of the patients who underwent hypothermia (32–34 °C) for 24 h had achieved independence at 6 months compared to 39% of those in the control group [34]. Of note, the target temperature was not specified in the control group in this study. Bernard et al. found similar results in a study that enrolled 77 comatose adults from VF OHCA from any etiology (no definition for coma was provided). Of the 43 patients who underwent hypothermia targeting 33 °C for 12 h, 49% had early favorable outcomes (discharge to home or rehabilitation) as opposed to 26% of the 34 patients in the normothermia group targeting 37 °C [35]. Both trials used cooling devices without a loop feedback mechanism, employed no control on the rate of rewarming, nor maintained normothermia following the cooling phase. Additionally, post-CA care and neuroprognostication were not standardized. After the publication of these trials, temperature control was quickly incorporated into guideline recommendations as standard post-CA care for adults suffering OHCA of shockable rhythms.
Evolution of Evidence Surrounding Temperature Control
Although the results of these two landmark trials were promising, there were several limitations that dampened the enthusiasm: they were open-label, their sample size the small with a high rate of screen failures (thus, raising concern for non-generalization), and at the time there were no established protocols for neuroprognostication post-CA and withdrawal of life-sustaining therapy (WLST) [36]. These limitations were subsequently addressed in the 2013 TTM1 trial, a RCT that analyzed 939 adult OHCA patients who remained unresponsive to verbal commands, and randomly assigned them to either temperature control at 33 °C or 36 °C for 24 h, followed by controlled active rewarming at 0.5 °C/h to 37 °C and subsequent normothermia until 72 h from randomization. Patients with a presumed cardiac etiology and any non-perfusing rhythm were eligible, excluding unwitnessed arrests with asystole as presenting rhythm. Standardized post-CA care was performed, including sedation and neuroprognostication, by a blinded independent physician using multimodal prognostication. The authors found no difference in survival or neurological outcomes at 6 months [37]. It is important to mention that the temperature in both arms was actively managed and no patient’s temperature was allowed to “self-regulate.” Unfortunately, within months of publication of the TTM1 trial, there was an abrupt drop in temperature control use altogether following CA in the United States, as well as decreased survival-to-discharge rates [38]. However, this reported decrease in survival rates could not be fully explained by the decline in temperature control use given the small number of hospitals used for the analysis. Post-CA care guidelines in 2015 started to recommend temperature control between 32 and 36 °C for at least 24 h in patients who remained comatose after OHCA or IHCA despite initial rhythm; however, data on the potential benefit of temperature control in patients who presented with an initial non-shockable rhythm (pulseless electrical activity (PEA) or asystole), or those who suffered IHCA, were lacking. Subsequently, a large retrospective study capturing the transition of target temperature during temperature control in a high-volume CA center demonstrated a negative impact on outcomes with shifting target temperatures from 33 to 36 °C [39].
In 2019, the HYPERION trial demonstrated that temperature control at 33 °C for at least 24 h in adults who suffered OHCA and IHCA with initial non-shockable rhythms increased favorable neurological outcomes at 90 days compared to targeted normothermia at 37 °C (10.2% vs. 5.7%) [40]. The study recruited 584 participants in France, but still had a high fragility index for this outcome. Two years later, the results of the largest and most rigorous RCT on temperature control to date, TTM2, challenged the routine use of target temperatures at 33 °C for OHCA of presumed cardiac etiology. The TTM2 trial recruited 1861 OHCA patients with shockable and non-shockable rhythms, and showed no difference in mortality or independence on ambulation at 6 months with targeted hypothermia at 33 °C versus targeted normothermia at < 37.5 °C [41]. Conversely, clinically significant arrhythmias were more common in the hypothermic group (24% vs. 16%). Since the publication of TTM2, there has been a debate on whether temperature control beyond merely preventing fever (i.e., ≤ 37.8 °C) during the first 72 h post-CA is enough to impact survival and neurological recovery [12, 13]. The TTM2 study had notable strengths, such as the largest sample size thus far, sound statistical methodology, blinding of families, statisticians, and outcome assessors, as well as established neuroprognostication protocols by an independent blinded physician only after 96 h post-randomization [12, 42]. Nonetheless, caution in extrapolating data from this trial to all CA populations is advised. In TTM2, over 90% of recruited patients experienced a witnessed CA and approximately 80% had bystander CPR [41]. These rates of witnessed CA and bystander CPR in the TTM2 population are remarkably higher than what is seen in clinical practice, with latest global data reporting that only 35–40% of patients who suffer OHCA receive bystander CPR [43,44,45]. Patients who do not receive bystander CPR and have longer “no-flow” times will generally present with more severe HIBI, and could potentially benefit the most from temperature control. However, the patients included in TTM2 had shorter “no-flow” times and higher rates of bystander-initiated CPR, potentially blunting the benefits of targeted hypothermia. Additionally, over 70% of recruited patients had shockable rhythms, cardiac or presumed cardiac etiology—with over 40% of ST elevation myocardial infarction—and only 28% presented with shock on admission; these facts demonstrate that the cohort is representative of a very specific subgroup of CA patients.
Most recently, the CAPITAL CHILL trial addressed the question of whether an even lower temperature target would be more beneficial for neurological recovery. The trial randomly assigned adult patients to a targeted temperature of 31 °C or 34 °C for 24 h, and demonstrated that a lower temperature goal of 31 °C did not improve outcomes compared to the standard of care targeted temperature of 34 °C. It is important to note that this was a single-center study and likely underpowered [46].
Another challenge interpreting this body of evidence is the inclusion of very broad patterns of brain injury leading to heterogeneity in the effects of temperature control at different targets and durations. As multiple authors have previously discussed, there are likely numerous brain injury phenotypes after CA, and neuroprotective interventions should likely be tailored to each individual brain injury target [47, 48]. If this differentiation of HIBI phenotypes is not carried out, therapies will likely fail as they are not being employed in the patient population most likely to benefit from them.
From Bench to Bedside: Integrating Temperature Control Evidence into Clinical Practice
Timing of Initiation
The post hoc analysis of the Continuous Chest Compressions trial showed that faster door-to-temperature control times were associated with better outcomes, particularly if within 122 min of admission. This trial supported the hypothesis that shorter times to temperature control initiation in OHCA patients increases the likelihood of a favorable neurological outcome [49]. However, given the retrospective nature of the study, as well as wide variability in temperature control initiation timing, cautious data interpretation is advised. Two RCTs have shown that pre-hospital cooling with cold saline infusion, as opposed to temperature control upon arrival to the treating hospital, confers no added benefit and, in fact, can cause a higher rate of early re-arrests [50, 51]. This is likely due to the decreased coronary perfusion caused by cold intravenous fluids [52], as well as pulmonary edema [50, 51]. Intra-arrest temperature control has been another area of interest, as pre-clinical studies in animal models showed promising results and, theoretically, prompt initiation of temperature control would mitigate even primary brain injury [32, 53,54,55,56]. Both the PRINCESS and RINSE trials showed that intra-arrest cooling in OHCA patients did not impact overall either neurological outcomes or survival rate, compared to temperature control after hospital admission, and this practice could even potentially decrease the rate of ROSC in initially shockable rhythms [52, 57]. However, a pooled analysis of these trials suggested a potential benefit of intra-arrest transnasal evaporative cooling in the subset of patients with shockable nonperfusing rhythms [58].
In a post hoc study of the TTM2 trial, no association of faster time to target 33 °C was noted with outcomes, challenging the critique that achievement of hypothermia earlier in the TTM2 trial could have altered the trial results [59].
Temperature control should be initiated as soon as possible after ROSC; however, there is insufficient data to support induction of pre-hospital cooling [9, 60].
Methods for Temperature Control
There are several pharmacologic and nonpharmacologic cooling methods employed to achieve target temperature. The most widely used are surface cooling devices, such as external gel pads containing cold water placed on the skin, and intravascular devices with a closed-loop feedback system that allow for more precise and controlled temperature control [36]. Infusion of cold saline intravenously is no longer recommended.
Due to their pragmatic nature, clinical trials in temperature control have been agnostic to the type of devices for temperature modulation. Several recent meta-analyses have demonstrated that intravascular cooling leads to better neurological outcomes compared to surface cooling, likely due to the intravascular route being more precise in maintaining the desired temperature, causing less temperature variation and being less associated with shivering [61, 62]. Several studies that evaluated surface cooling did not indicate whether temperature feedback was utilized [62]. It is also important to consider the possible complications of each method. The most common adverse events associated with surface cooling are skin injuries, such as tears and decubitus ulcers, whereas thrombosis, hemorrhage, and infections are prominent in intravascular cooling, as expected with central access [63,64,65]. While patient-specific factors could influence the selection of the method for temperature control (e.g., those with higher risk for thrombotic complications could benefit from external pads), most commonly the decision hinges upon the availability of the method in each institution.
Temperature Target Selection
Although it is imperative to actively control temperature and prevent fever in post-CA patients, the ideal target temperature for each CA phenotype has yet to be elucidated. It is quite possible that in the future individual factors leading to distinct injury phenotypes may influence the ideal temperature target [50, 66, 67]. In fact, five distinct phenotypes of post-CA brain injury were identified in a large retrospective cohort using unsupervised machine learning[47]. These phenotypes were determined based on features of neurological exam, EEG, and neuroimaging. The heterogeneity that exists within this patient population is reflective of the need for personalization of resuscitation efforts, such as temperature control strategies. However, more research is needed to better elucidate these findings, and to support the recommendation of different target temperatures for specific phenotypes.
While guidelines recommend a target temperature of 32–37.5 °C for at least 24 h after ROSC, there is no set temperature that has been proven to show more benefit over another, and the selection tends to be patient-dependent. A recent Bayesian meta-analysis determined that temperature control at 32–34 °C as compared to temperatures over 36 °C did not lead to more positive neurological outcomes [68]. Furthermore, a network meta-analysis indicated that hypothermia at 31–36 °C compared to normothermia at 37–37.8 °C did not lead to increased survival rates or better outcomes, and was associated with a greater risk of developing arrhythmias [14]. Prior data suggest that a target temperature ~ 36 °C might be preferrable in those with bleeding, intracerebral hemorrhage, or hemodynamic compromise, all of which could be exacerbated by lower temperatures [7]. Furthermore, rewarming patients who are spontaneously hypothermic may have detrimental effects, and targeting lower temperatures to avoid rewarming may be considered[50]. A lower temperature of 33 °C may be desired in those at risk for worse neurological damage from severe HIBI [4, 50, 67], but may also be associated with increased risk of arrhythmias in patients with cardiac etiologies for CA.
Maintenance Phase
Once temperature control is initiated, core temperature should be measured using either an esophageal, bladder, or intravascular probe. It is discouraged to use a rectal, oral, or axillary probe, as core temperature measurements are not as accurate. As recommended by guidelines, the cooling phase (a constant temperature between 32 and 37.5 °C) should last at least 24 h after ROSC, with special attention to avoidance of fever (> 37.7 °C) for at least > 72 h after ROSC. In the TH48 trial, extending temperature control at 33 °C for 48 h conferred no additional benefit over 24 h [69] on neurological outcomes at 6 months. The extended duration arm was associated with increased adverse events (although most of these appeared to be mild) as well as longer ICU length of stay. Until further data from large RCTs are obtained, target temperatures should be maintained for 24 h.
Rewarming Phase
The rewarming phase should occur at a rate of 0.15–0.25 °C/h, as rapid rewarming could cause an increased inflammatory response and potentially worsen outcomes [70, 71]. However, these rates of rewarming are extrapolated from study protocols from RCTs in temperature control evaluating target temperatures, and not based on large trials comparing different arms with rates of rewarming. To date, only ISOCRATE has explored the potential impact of slower rewarming rates, in a pilot study that failed to demonstrate a change in IL-6 and neurofilament light chain levels with 0.25 °C/h compared to 0.5 °C/h [72]. Hence, the ERC/ESICM guidelines make no recommendations on a specific rewarming rate [73]. Attention to fever prevention must be paid for at least 48 h after finalization of rewarming, and for at least 72 h after ROSC as general practice. However, a Danish RCT showed that maintaining normothermia for over 48 h, when compared to 12 h following rewarming from initial temperature control at 36 °C, failed to confer any benefit in reducing mortality or significant disability [74]. Temperature fluctuations, if not actively controlled, can be extremely labile and ultimately detrimental to outcomes [48].
Complications of TTM and Management Strategies
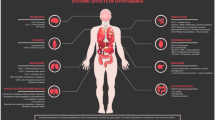
The physiologic alterations of temperature control and its associated adverse effects are largely dependent on the depth of temperature control, or how cold the target temperature is (Fig. 1). This is in conjunction with the patients’ baseline comorbidities, age, organ dysfunction, and several other factors that may contribute to the wide array of complications from hypothermia on nearly every organ system, a few of which are discussed below.
Shivering
Shivering is one of the most frequent adverse effects of temperature control, occurring in up to 40% of patients [75]. Normothermia is a tightly regulated process in the human body maintained through central and peripheral pathways. Thermoregulation starts with the hypothalamus, which transmits sensory information to the peripheral nervous system, including the skin, tissues, and organs, to elicit thermoregulatory response [76]. The drop in core body temperatures below 36 °C stimulates this innate thermoregulatory response inducing peripheral vasoconstriction and shivering to increase heat production [76, 77]. Shivering can impede the cooling process and negate the therapeutic benefit of temperature control, increase cerebral oxygen consumption, raise intracranial pressure, and decrease brain tissue oxygen tension [78]. Thus, implementation of standardized protocols to prevent, and promptly abort, shivering is critical to ensure that the benefits of temperature control are achieved.
The Bedside Shiver Assessment Scale (BSAS) is the most frequently employed, validated tool to assess shivering and the impact of therapeutic interventions [79]. This scoring system ranges from 0 to 3 (0 = no shivering to 3 = severe shivering), grading severity based on evaluation of muscle movements; the goal BSAS score is < 1.
Shivering management should entail a tiered approach, including pharmacologic and non-pharmacologic interventions based on hourly BSAS scores and patient-specific factors [79, 80]. Skin temperature is estimated to contribute 20% to the control of shivering, and it has been shown that, for every 4 °C increase in skin temperature, a 1 °C decrease in shivering threshold can be achieved [81, 82]. Non-pharmacologic interventions focused on this mechanism, including cutaneous counter-warming techniques (in particular to extremities, such as hands and feet), head wrapping, or humidified air inhalation, have all been shown to reduce shivering, oxygen consumption, and resting energy expenditure [79].
Pharmacologic management of shivering involves a multimodal, tiered approach with agents, such as antipyretics, sedation, analgesia, and neuromuscular blockade, and adjunctive therapies, such as buspirone and magnesium [80]. Impaired thermoregulatory responses associated with brain injury often render antipyretics ineffective for fever control; however, initiation of acetaminophen remains commonly used in practice with an estimated fever control of 0.2 °C [83]. Magnesium infusions suppress shivering through vasodilation and direct reduction muscle tone, with an estimated efficacy in reducing the shivering threshold of 0.3 °C. Sedatives employed for shivering control include dexmedetomidine, benzodiazepines, propofol, and buspirone, with shiver threshold reduction ranging from 0.5 to 2.4 °C [79]. Of analgesics, meperidine appears to have the greatest advantage of reducing the shivering threshold by 1.2–2.4 °C; fentanyl is also efficacious due to its neutral hemodynamic effects [79, 80]. Neuromuscular blockade agents are the most effective strategy, and may be used in patients with deep sedation if shivering cannot be promptly controlled with the above measures. In fact, the liberal, short-term use of neuromuscular blockade during temperature control in CA has been associated with a favorable impact on outcomes in a meta-analysis of three RCT and nine observational studies. The Columbia Anti-Shivering protocol provides a tiered strategy for shivering management, utilizing a combination of the above pharmacotherapies in relation to the BSAS [80].
Cardiovascular Effects
At the initiation of temperature control, tachycardia and hypertension occur in response to cutaneous vasoconstriction and shivering to preserve core body temperature. Hypothermia-induced vasoconstriction can increase central venous pressure and systemic vascular resistance, with an estimated increased systolic blood pressure by 10 mmHg. As temperatures continue to decline, cardiac output can decrease by 25–40% at 32–34 °C, primarily due to a reduction in heart rate and cardiac contractility [84]. When body temperature declines below 35 °C, sinus bradycardia can occur due to decreased sinoatrial node depolarization. Electrolyte imbalances resulting in hypokalemia, hypomagnesemia, hypocalcemia, and pH alterations during temperature control may further contribute to the arrhythmogenic state. The resultant electrocardiographic findings may include prolonged PR, QT, and QRS intervals [85]. QTc prolongation has also been seen during hypothermia; however, no data suggest an increased risk of torsade de pointes [86]. More severe or even fatal arrythmias, including atrial fibrillation, ventricular fibrillation, and ventricular tachycardia, remain rare when core body temperature remains > 30 °C [84]. Hypotension can develop due to cardiac dysfunction or intravascular volume depletion from hypothermia-induced cold diuresis. Hypotension is also common during the rewarming phase due to the reversal of hypothermia-induced vasoconstriction.
Management of cardiovascular complications during hypothermia is critical to mitigate the risk of cerebral hypoperfusion. Medical interventions with vasopressor and inotropic support along with fluid resuscitation remain standard measures in the management of hypotension. Bradycardia in the setting of hypothermia often does not require treatment unless complicated by hemodynamic instability. Atropine is ineffective for hypothermia-induced bradycardia and transcutaneous pacing, or a pacemaker may need to be considered [84, 86]. In the event of a dysrhythmia, it is important to note that hypothermia may impair the efficacy of anti-arrhythmic agents, therefore consideration to increase the target temperature may be necessary in the setting of arrhythmias.
Coagulation
Hypothermia-induced coagulopathy is characterized by increasing bleeding time secondary to a decrease in the function and production of platelets, endothelial dysfunction, and impaired kinetics of coagulation factors and enzymes involved in the coagulation cascade. Temperatures < 35 °C have the greatest effect on platelet function and coagulation parameters, resulting in prolongation of the prothrombin time and partial thromboplastin time [84, 87]. Platelet function is not only suppressed but may also be sequestered in the spleen and liver during the rewarming process [88]. Although coagulation parameters are altered during hypothermia, data from RCTs of temperature control, including in those with intracranial hemorrhage and traumatic brain injury, have not suggested an increased rate of significant bleeding [79, 89, 90]. Thromboelastography is suggested as the optimal method to characterize coagulopathy during hypothermia [79, 90]. The susceptibility to bleeding during the induction and maintenance phase, however, should be considered in those at high risk, and non-essential procedures and/or interventions that may heighten this risk should be deferred, if possible.
Infection
Inhibition of the inflammatory response is one of the proposed mechanisms of the neuroprotective benefit of temperature control; however, untoward consequences on the immune system may heighten the risk of infection. Defense mechanisms of the innate immune system are impaired during hypothermia, with data illustrating attenuated chemotactic, phagocytic, and apoptotic activity of leukocytes at 33 °C compared to 37 °C [91]. Hypothermia also impairs production of proinflammatory cytokines, resulting in decreased leukocyte migration and phagocytosis [91]. Hyperglycemia and electrolyte derangements during hypothermia only further heighten the infection risk [87].
The estimated risk of infectious complications has been reported as high as 67%, with the duration of hypothermia as a critical factor increasing this risk [87, 92]. A systematic review and meta-analysis of controlled trials of temperature control found a higher prevalence of pneumonia (RR 1.44, CI 1.1–1.9) and sepsis (RR 1.8, CI 1.4–3.1) among the hypothermia cohort compared to control [93]. Staphylococcus aureus has been identified as the main causative pathogen among CA survivors [92]. Accurate and prompt detection of infection during hypothermia remains an important challenge, as febrile episodes are suppressed and biomarkers such as procalcitonin and C-reactive protein have limited utility after CA [94]. Although prophylactic antibiotics have been shown to reduce the incidence of ventilator-associated pneumonia in patients cooled to 32–34 °C, no significant differences in mortality or ventilator-free days have been observed [95]. The lack of benefit in patient-centered clinical outcomes and concern for drug resistance and adverse effects of antimicrobial therapy limit the implementation of prophylactic antibiotics in clinical practice.
Routine assessment of blood and sputum cultures during the hypothermia phase has been suggested, with initiation of antimicrobials in those with positive cultures or radiographic findings concerning for infection. Other features of infection may include increased requirements from the cooling device to maintain hypothermia, which may indicate fever generation in the presence of an infection. Hypothermia-induced vasoconstriction can also heighten the risk of wound infections, and the development of bedsores from immobilization can ensue. Careful attention and precautionary measures to minimize the risk of wound complications, and special attention and care to catheters and central lines, should be implemented.
Metabolic and Electrolyte Derangements
Severe electrolyte disturbances, including hypokalemia, hypomagnesemia, hypophosphatemia, and hypocalcemia, may occur during the cooling phase, which may lead to severe physiologic complications, such as cardiac arrythmias, diaphragmatic weakness, and impaired coagulation. The mechanisms of electrolyte imbalances during hypothermia include intracellular shift and cold-induced diuresis, which subsequently result in renal excretion of potassium, magnesium, and phosphate [96]. During the rewarming phase, however, these processes reverse, and subsequent extracellular shifts of these electrolytes ensue. Serum levels of magnesium, phosphate, and calcium should remain within normal ranges. Potassium should be closely monitored, and supplementation is suggested to maintain a serum potassium of 3.0–3.5 mEq/L during the induction and maintenance phase when targeting lower temperatures to mitigate the risk of rebound hyperkalemia during rewarming [79].
Suppression of the cerebral metabolic rate with hypothermia subsequently affects all other organ systems and their associated physiologic functions. Pancreatic insulin production and secretion is impaired during hypothermia, posing a risk of hyperglycemia [97]. Hyperglycemia is harmful to the injured brain, and tight blood glucose is essential to mitigate harmful neurologic sequalae, infection risk, and other complications. During the rewarming phase, as the body restores its metabolic functions, hypoglycemia can occur that, if severe, can result in seizures, coma, and even death. Tight blood glucose control utilizing insulin infusions should be employed to target a serum blood glucose of 140–180 mEq/L, minimizing the risk of hypo- and hyperglycemia. Other metabolic disturbances can include metabolic acidosis, secondary to increased production of ketones, lactate, and glycerol.
Respiratory Effects
Minute ventilation requirements may fluctuate drastically during temperature control, given changes in metabolic rate and carbon dioxide production with different core temperatures; thus, attention must be paid to serial arterial blood gas values during induction, maintenance, and rewarming. Blood gas values, however, are affected by blood temperature, and therefore it is critical to ensure that arterial blood gas measurements are temperature-corrected prior to analysis [79]. This is important, as hypocarbia has been associated with worsened brain injury [98], and serial adjustment of minute ventilation to match the target of normocarbia (PaCO2 ~ 40 mmHg) is key.
Emerging evidence supports potential benefit of hypercarbia in post-CA, as demonstrated by the augmentation of cerebral blood flow, decrease in oxidative brain injury, and downregulation of apoptosis and autophagy in mural models of asphyxia CA subjected to inhaled carbon dioxide [99]. The Targeted Therapeutic Mild Hypercapnia after Resuscitated Cardiac Arrest (TAME) trial is currently ongoing and set to randomly allocate 1700 subjects to permissive hypercarbia (PaCO2 50–55 mmHg) or to standard care (PaCO2 35–45 mmHg); the results of this trial will be highly informative and guide the ideal ventilatory targets in the post-CA period.
Drug Pharmacokinetics and Pharmacodynamics
Consideration of the impact of therapeutic hypothermia on the pharmacokinetics (PK) and pharmacodynamics (PD) of drugs administered in the intensive care unit is critical in the care of patients undergoing temperature control. Hepatic drug metabolism is significantly reduced during hypothermia, resulting in accumulation of medications metabolized via cytochrome P450 (CYP450) enzymes and a potential for drug toxicity. For example, midazolam and fentanyl, commonly employed for sedation and analgesia during temperature control, undergo extensive metabolism via CYP3A4. A pharmacokinetic study of mice administered midazolam and fentanyl as a continuous infusion demonstrated 17.5% and 20.5% reductions in systemic clearance, respectively, when cooled to 33 °C compared to normothermia [100]. In a study of 14 patients undergoing mild hypothermia treated with phenytoin demonstrated a 180% increase in the area under the curve, with a corresponding 67% reduction in systemic clearance [101]. Notably, supratherapeutic phenytoin concentrations remained even after the rewarming phase, making it critical to monitor concentrations of narrow therapeutic index medications during temperature control, and in the post-rewarming phase, to ensure adequate dose adjustments to minimize toxic exposures.
In contrast, medications that are prodrugs and require metabolism via CYP450s to their active metabolite to exert their therapeutic effect (e.g., clopidogrel) may have reduced pharmacodynamic efficacy during temperature control. In a study of CA patients secondary to acute coronary syndrome undergoing temperature control treated with clopidogrel (n = 25), whole blood samples measuring platelet reactivity of clopidogrel demonstrated a 0% response rate after a 300-mg loading dose, and a mere 5% on day 3 after initiation of maintenance dosing [102]. Other important PK/PD alterations during temperature control may include altered drug absorption, increased clearance of hydrophilic drugs secondary to cold-induced diuresis, and changes in drug transport and altered volume of distribution, which may vary depending on the phase of therapy, highlighting the critical importance of drug dosing and monitoring in patients undergoing temperature control [103].
Multimodal Prognostication and Timing Around Temperature Control
Neuroprognostication after CA is a challenging and complex process with many variables affecting its optimal timing. Current guidelines recommend delayed and multimodal prognostication; that is, using multiple ancillary tests in addition to clinical examination to get a better picture of neurological function and possible recovery [9]. The reason behind multimodal prognostication is the dire need to avoid self-fulfilling prophecy bias. This happens when the treating physician is aware of the results of neuroprognostic tests being investigated, and bases further management, including WLST, on these results [104,105,106], with consequent overinflation of their prediction performance. Around 80% of patients who survive CA will remain comatose, and most of them will die from WLST [6, 107]. Predicting neurological outcome in HIBI through a multimodal approach gives surrogate decision-makers realistic expectations, aids in identifying which critical care resources should be mobilized, and prevents early WLST due to perceived poor neurological prognosis (WLST-N) [107, 108]. One-third of WLST-N occurs < 72 h after ROSC, suggesting that ~ 1500 people a year in the United States could have potentially recovered if premature WLST-N had not occurred [108].
Neuroprognostication should be delayed for at least 72 h after ROSC, and, if temperature control was employed, it is sometimes necessary to delay it for several days after active temperature control has ended. Drug metabolism decreases during targeted hypothermia, and sedatives along with neuromuscular blockade may not be cleared, yet allow for an unconfounded neurological exam [105, 107, 109].
Future Trials
Despite robust efforts in the published trials to establish the efficacy of temperature control post-CA, uncertainty remains on the optimal temperature that would result in the greatest improvement in neurologic outcomes. Ongoing preclinical and clinical research in therapeutic hypothermia is aimed at evaluating the efficacy of hypothermia, the ideal temperature, duration, method of cooling, potential adjuncts for neuroprotection, neuroprognostication, and more. Given the degree of heterogeneity in current trials investigating temperature control, the goal of ongoing trials is on precision care and how to best tailor hypothermia intervention to the specific patient characteristics. The Influence of Cooling Duration on Efficacy in Cardiac Arrest Patients (ICECAP trial) [110] is an ongoing multi-center, response-adaptative dose-finding trial investigating the optimal duration of induced hypothermia, ranging from 6 to 72 h, that is associated with neuroprotection among survivors of CA. This study is actively recruiting in tandem with the Precision Care in Cardiac Arrest: Influence of Cooling duration on Efficacy in Cardiac Arrest Patients [110] trial, designed using machine learning to discover novel biomarkers to predict the duration of hypothermia and associated functional outcomes [110]. ICECAP, which has an estimated completion date of 2025, aims to create a dose–response curve for the duration of temperature control using frequently collected, multimodality data points. This trial has the potential to identify the patient populations that will benefit the most and least from longer and shorter durations of temperature control, but will not address which temperature should be targeted. Research focused on personalized care will allow for adjustments of therapy based on individualized needs and therapeutic response. In pre-clinical data, several promising novel brain injury biomarkers to predict clinical outcomes after CA continue to be investigated, including neurofilament light, ubiquitin carboxy-terminal hydro-lase L1, glial fibrillary acidic protein, and tau protein. Selective brain cooling has emerged as a promising strategy to attain the neuroprotective benefits of hypothermia while minimizing the myriad of system complications from whole body cooling [111]. Several adjunctive agents for neuroprotection remain in active research and include high-dose erythropoietin, melatonin, inhaled gases such as xenon, insulin-like growth factor, and magnesium [112]. While these agents have demonstrated neuroprotective benefits, the role in conjunction with hypothermia remains to be explored.
Conclusions
Though temperature control to 32–37.5 °C is the current standard of care, the quality of the data is low–moderate and does not show benefit in improving neurological outcomes or survival rates. Because of this, several centers will likely abandon temperature control altogether or shift their practice to a more lenient temperature control [48], possibly focusing on fever prevention with less reliable strategies (i.e., antipyretics and intermittent temperature readings). It is important to acknowledge that the TTM2 trial incorporated a rigorous bundle of post-CA care with strict temperature control, which required use of cooling devices in nearly half of recruited patients to the fever-prevention arm. Moreover, the implementation of high-quality temperature control is paramount, as deficiencies in so much as one element (i.e., timing of initiation, temperature selection, fluctuation in temperature, use of analgosedation, shivering suppression, hemodynamic support, among others) can affect outcomes and cloud results. Future RCTs will likely focus on high-quality active targeted normothermia and its benefits on outcomes, compared to targeted hypothermia.
References
Tsao CW, Aday AW, Almarzooq ZI, Alonso A, Beaton AZ, Bittencourt MS, et al. Heart Disease and Stroke Statistics-2022 update: a report from the American Heart Association. Circulation. 2022;145(8):e153–639.
Yin L, Xie D, He D, Chen Z, Guan Y, Wang J, et al. Survival to hospital discharge and neurological outcomes with targeted temperature management after in-hospital cardiac arrest: a systematic review and meta-analysis. Ann Palliat Med. 2022;11(1):68–76.
Shrestha DB, Sedhai YR, Budhathoki P, Gaire S, Adhikari A, Poudel A, et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest: a systematic review and meta-analysis of randomized controlled trials. Ann Med Surg (Lond). 2022;74: 103327.
Nolan JP, Abella BS. Postresuscitation care and prognostication. Curr Opin Crit Care. 2021;27(6):649–55.
Coute RA, Nathanson BH, Panchal AR, Kurz MC, Haas NL, McNally B, et al. Disability-adjusted life years following adult out-of-hospital cardiac arrest in the United States. Circ Cardiovasc Qual Outcomes. 2019;12(3): e004677.
Sandroni C, Cronberg T, Sekhon M. Brain injury after cardiac arrest: pathophysiology, treatment, and prognosis. Intensive Care Med. 2021;47(12):1393–414.
Taccone FS, Picetti E, Vincent JL. High quality Targeted Temperature Management (TTM) after cardiac arrest. Crit Care. 2020;24(1):6.
Lemiale V, Dumas F, Mongardon N, Giovanetti O, Charpentier J, Chiche JD, et al. Intensive care unit mortality after cardiac arrest: the relative contribution of shock and brain injury in a large cohort. Intensive Care Med. 2013;39(11):1972–80.
Nolan JP, Sandroni C, Bottiger BW, Cariou A, Cronberg T, Friberg H, et al. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines 2021: post-resuscitation care. Resuscitation. 2021;161:220–69.
Panchal AR, Bartos JA, Cabanas JG, Donnino MW, Drennan IR, Hirsch KG, et al. Part 3: adult basic and advanced life support: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(16_suppl_2):S366–468.
Annoni F, Peluso L, Fiore M, Nordberg P, Svensson L, Abella B, et al. Impact of therapeutic hypothermia during cardiopulmonary resuscitation on neurologic outcome: a systematic review and meta-analysis. Resuscitation. 2021;162:365–71.
Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Belohlavek J, Callaway C, et al. Targeted hypothermia versus targeted Normothermia after out-of-hospital cardiac arrest (TTM2): a randomized clinical trial-Rationale and design. Am Heart J. 2019;217:23–31.
Elbadawi A, Sedhom R, Baig B, Mahana I, Thakker R, Gad M, et al. Targeted hypothermia vs. targeted normothermia in survivors of cardiac arrest: a systematic review and meta-analysis of randomized trials. Am J Med. 2021. https://doi.org/10.1016/j.amjmed.2021.11.014.
Fernando SM, Di Santo P, Sadeghirad B, Lascarrou JB, Rochwerg B, Mathew R, et al. Targeted temperature management following out-of-hospital cardiac arrest: a systematic review and network meta-analysis of temperature targets. Intensive Care Med. 2021;47(10):1078–88.
Matory AL, Alkhachroum A, Chiu WT, Eliseyev A, Doyle K, Rohaut B, et al. Electrocerebral signature of cardiac death. Neurocrit Care. 2021;35(3):853–61.
Sekhon MS, Ainslie PN, Griesdale DE. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: a “two-hit” model. Crit Care. 2017;21(1):90.
Hartings JA, Shuttleworth CW, Kirov SA, Ayata C, Hinzman JM, Foreman B, et al. The continuum of spreading depolarizations in acute cortical lesion development: examining Leao’s legacy. J Cereb Blood Flow Metab. 2016. https://doi.org/10.1177/0271678X16654495.
Kaminogo M, Suyama K, Ichikura A, Onizuka M, Shibata S. Anoxic depolarization determines ischemic brain injury. Neurol Res. 1998;20(4):343–8.
Fischer M, Bockhorst K, Hoehn-Berlage M, Schmitz B, Hossmann K-A. Imaging of the apparent diffusion coefficient for the evaluation of cerebral metabolic recovery after cardiac arrest. Magn Reson Imaging. 1995;13(6):781–90.
Li L, Poloyac SM, Watkins SC, St Croix CM, Alexander H, Gibson GA, et al. Cerebral microcirculatory alterations and the no-reflow phenomenon in vivo after experimental pediatric cardiac arrest. J Cereb Blood Flow Metab. 2019;39(5):913–25.
Elmer J, Callaway CW. The brain after cardiac arrest. Semin Neurol. 2017;37(1):19–24.
Buunk G. Cerebral blood flow after cardiac arrest. Neth J Med. 2000;57(3):106–12.
Zeiner A, Holzer M, Sterz F, Schorkhuber W, Eisenburger P, Havel C, et al. Hyperthermia after cardiac arrest is associated with an unfavorable neurologic outcome. Arch Intern Med. 2001;161(16):2007–12.
Leary M, Grossestreuer AV, Iannacone S, Gonzalez M, Shofer FS, Povey C, et al. Pyrexia and neurologic outcomes after therapeutic hypothermia for cardiac arrest. Resuscitation. 2013;84(8):1056–61.
Gebhardt K, Guyette FX, Doshi AA, Callaway CW, Rittenberger JC, Arrest PC, S. Prevalence and effect of fever on outcome following resuscitation from cardiac arrest. Resuscitation. 2013;84(8):1062–7.
Sekhon MS, Smielewski P, Bhate TD, Brasher PM, Foster D, Menon DK, et al. Using the relationship between brain tissue regional saturation of oxygen and mean arterial pressure to determine the optimal mean arterial pressure in patients following cardiac arrest: a pilot proof-of-concept study. Resuscitation. 2016;106:120–5.
Geocadin RG, Wijdicks E, Armstrong MJ, Damian M, Mayer SA, Ornato JP, et al. Practice guideline summary: reducing brain injury following cardiopulmonary resuscitation: Report of the guideline development, dissemination, and implementation subcommittee of the American Academy of Neurology. Neurology. 2017;88(22):2141–9.
Dietrichs ES, Dietrichs E. Neuroprotective effects of hypothermia. Tidsskr Nor Laegeforen. 2015;135(18):1646–51.
Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci. 2012;13(4):267–78.
Williams GR Jr, Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg. 1958;148(3):462–8.
Benson DW, Williams GRJ, Spencer FC, Yates AJ. The, use of hypothermia after cardiac arrest. Anesth Analg. 1959;38(6):423–8.
Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, et al. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab. 1990;10(1):57–70.
Sterz F, Safar P, Tisherman S, Radovsky A, Kuboyama K, Oku K. Mild hypothermic cardiopulmonary resuscitation improves outcome after prolonged cardiac arrest in dogs. Crit Care Med. 1991;19(3):379–89.
Hypothermia after Cardiac Arrest Study G. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med. 2002;346(8):549–56.
Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, et al. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med. 2002;346(8):557–63.
Rasmussen TP, Bullis TC, Girotra S. Targeted temperature management for treatment of cardiac arrest. Curr Treat Options Cardiovasc Med. 2020;22(11):39.
Nielsen N, Wetterslev J, Cronberg T, Erlinge D, Gasche Y, Hassager C, et al. Targeted temperature management at 33 degrees C versus 36 degrees C after cardiac arrest. N Engl J Med. 2013;369(23):2197–206.
Bradley SM, Liu W, McNally B, Vellano K, Henry TD, Mooney MR, et al. Temporal trends in the use of therapeutic hypothermia for out-of-hospital cardiac arrest. JAMA Netw Open. 2018;1(7): e184511.
Johnson NJ, Danielson KR, Counts CR, Ruark K, Scruggs S, Hough CL, et al. Targeted temperature management at 33 versus 36 degrees: a retrospective cohort study. Crit Care Med. 2020;48(3):362–9.
Lascarrou JB, Merdji H, Le Gouge A, Colin G, Grillet G, Girardie P, et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N Engl J Med. 2019;381(24):2327–37.
Dankiewicz J, Cronberg T, Lilja G, Jakobsen JC, Levin H, Ullen S, et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N Engl J Med. 2021;384(24):2283–94.
Lilja G, Nielsen N, Ullen S, Blennow Nordstrom E, Dankiewicz J, Friberg H, et al. Protocol for outcome reporting and follow-up in the targeted hypothermia versus targeted normothermia after out-of-hospital cardiac arrest trial (TTM2). Resuscitation. 2020;150:104–12.
Dainty KN, Colquitt B, Bhanji F, Hunt EA, Jefkins T, Leary M, et al. Understanding the importance of the lay responder experience in out-of-hospital cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2022;145(17):e852–67.
Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, et al. Heart disease and stroke statistics-2021 update: a report from the American Heart Association. Circulation. 2021;143(8):e254–743.
Soar J, Berg KM, Andersen LW, Böttiger BW, Cacciola S, Callaway CW, et al. Adult advanced life support: 2020 international consensus on cardiopulmonary resuscitation and emergency cardiovascular care science with treatment recommendations. Resuscitation. 2020;156:A80–119.
Le May M, Osborne C, Russo J, So D, Chong AY, Dick A, et al. Effect of moderate vs. mild therapeutic hypothermia on mortality and neurologic outcomes in comatose survivors of out-of-hospital cardiac arrest: the CAPITAL CHILL randomized clinical trial. JAMA. 2021;326(15):1494–503.
Elmer J, Coppler PJ, May TL, Hirsch K, Faro J, Solanki P, et al. Unsupervised learning of early post-arrest brain injury phenotypes. Resuscitation. 2020;153:154–60.
Taccone FS, Lascarrou JB, Skrifvars MB. Targeted temperature management and cardiac arrest after the TTM-2 study. Crit Care. 2021;25(1):275.
Stanger D, Kawano T, Malhi N, Grunau B, Tallon J, Wong GC, et al. Door-to-targeted temperature management initiation time and outcomes in out-of-hospital cardiac arrest: insights from the continuous chest compressions trial. J Am Heart Assoc. 2019;8(9): e012001.
Kim F, Nichol G, Maynard C, Hallstrom A, Kudenchuk PJ, Rea T, et al. Effect of prehospital induction of mild hypothermia on survival and neurological status among adults with cardiac arrest: a randomized clinical trial. JAMA. 2014;311(1):45–52.
Bernard SA, Smith K, Cameron P, Masci K, Taylor DM, Cooper DJ, et al. Induction of therapeutic hypothermia by paramedics after resuscitation from out-of-hospital ventricular fibrillation cardiac arrest: a randomized controlled trial. Circulation. 2010;122(7):737–42.
Nordberg P, Taccone FS, Truhlar A, Forsberg S, Hollenberg J, Jonsson M, et al. Effect of trans-nasal evaporative intra-arrest cooling on functional neurologic outcome in out-of-hospital cardiac arrest: the PRINCESS randomized clinical trial. JAMA. 2019;321(17):1677–85.
Abella BS, Zhao D, Alvarado J, Hamann K, Vanden Hoek TL, Becker LB. Intra-arrest cooling improves outcomes in a murine cardiac arrest model. Circulation. 2004;109(22):2786–91.
Nozari A, Safar P, Stezoski SW, Wu X, Kostelnik S, Radovsky A, et al. Critical time window for intra-arrest cooling with cold saline flush in a dog model of cardiopulmonary resuscitation. Circulation. 2006;113(23):2690–6.
Zhao D, Abella BS, Beiser DG, Alvarado JP, Wang H, Hamann KJ, et al. Intra-arrest cooling with delayed reperfusion yields higher survival than earlier normothermic resuscitation in a mouse model of cardiac arrest. Resuscitation. 2008;77(2):242–9.
Wang H, Barbut D, Tsai MS, Sun S, Weil MH, Tang W. Intra-arrest selective brain cooling improves success of resuscitation in a porcine model of prolonged cardiac arrest. Resuscitation. 2010;81(5):617–21.
Bernard SA, Smith K, Finn J, Hein C, Grantham H, Bray JE, et al. Induction of therapeutic hypothermia during out-of-hospital cardiac arrest using a rapid infusion of cold saline: the RINSE Trial (Rapid Infusion of Cold Normal Saline). Circulation. 2016;134(11):797–805.
Taccone FS, Hollenberg J, Forsberg S, Truhlar A, Jonsson M, Annoni F, et al. Effect of intra-arrest trans-nasal evaporative cooling in out-of-hospital cardiac arrest: a pooled individual participant data analysis. Crit Care. 2021;25(1):198.
Simpson RFG, Dankiewicz J, Karamasis GV, Pelosi P, Haenggi M, Young PJ, et al. Speed of cooling after cardiac arrest in relation to the intervention effect: a sub-study from the TTM2-trial. Crit Care. 2022;26(1):356.
Merchant RM, Topjian AA, Panchal AR, Cheng A, Aziz K, Berg KM, et al. Part 1: executive summary: 2020 American Heart Association Guidelines for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation. 2020;142(162):S337–57.
Matsumoto S, Kuno T, Mikami T, Takagi H, Ikeda T, Briasoulis A, et al. Effect of cooling methods and target temperature on outcomes in comatose patients resuscitated from cardiac arrest: systematic review and network meta-analysis of randomized trials. Am Heart J. 2022;256:73–84.
Ramadanov N, Arrich J, Klein R, Herkner H, Behringer W. Intravascular versus surface cooling in patients resuscitated from cardiac arrest: a systematic review and network meta-analysis with focus on temperature feedback. Crit Care Med. 2022;50(6):999–1009.
Jarrah S, Dziodzio J, Lord C, Fraser GL, Lucas L, Riker RR, et al. Surface cooling after cardiac arrest: effectiveness, skin safety, and adverse events in routine clinical practice. Neurocrit Care. 2011;14(3):382–8.
Maze R, Le May MR, Froeschl M, Hazra SK, Wells PS, Osborne C, et al. Endovascular cooling catheter related thrombosis in patients undergoing therapeutic hypothermia for out of hospital cardiac arrest. Resuscitation. 2014;85(10):1354–8.
Pittl U, Schratter A, Desch S, Diosteanu R, Lehmann D, Demmin K, et al. Invasive versus non-invasive cooling after in- and out-of-hospital cardiac arrest: a randomized trial. Clin Res Cardiol. 2013;102(8):607–14.
Callaway CW, Coppler PJ, Faro J, Puyana JS, Solanki P, Dezfulian C, et al. Association of initial illness severity and outcomes after cardiac arrest with targeted temperature management at 36 degrees C or 33 degrees C. JAMA Netw Open. 2020;3(7): e208215.
Nishikimi M, Ogura T, Nishida K, Hayashida K, Emoto R, Matsui S, et al. Outcome related to level of targeted temperature management in postcardiac arrest syndrome of low, moderate, and high severities: a nationwide multicenter prospective registry. Crit Care Med. 2021;49(8):e741–50.
Aneman A, Frost S, Parr M, Skrifvars MB. Target temperature management following cardiac arrest: a systematic review and Bayesian meta-analysis. Crit Care. 2022;26(1):58.
Kirkegaard H, Soreide E, de Haas I, Pettila V, Taccone FS, Arus U, et al. Targeted temperature management for 48 vs. 24 hours and neurologic outcome after out-of-hospital cardiac arrest: a randomized clinical trial. JAMA. 2017;318(4):341–50.
Hifumi T, Inoue A, Kokubu N, Hase M, Yonemoto N, Kuroda Y, et al. Association between rewarming duration and neurological outcome in out-of-hospital cardiac arrest patients receiving therapeutic hypothermia. Resuscitation. 2020;146:170–7.
Cho E, Lee SE, Park E, Kim HH, Lee JS, Choi S, et al. Pilot study on a rewarming rate of 0.15 degrees C/hr versus 0.25 degrees C/hr and outcomes in post cardiac arrest patients. Clin Exp Emerg Med. 2019;6(1):25–30.
Lascarrou J-B, Guichard E, Reignier J, Le Gouge A, Pouplet C, Martin S, et al. Impact of rewarming rate on interleukin-6 levels in patients with shockable cardiac arrest receiving targeted temperature management at 33 °C: the ISOCRATE pilot randomized controlled trial. Crit Care. 2021. https://doi.org/10.1186/s13054-021-03842-9.
Sandroni C, Nolan JP, Andersen LW, Bottiger BW, Cariou A, Cronberg T, et al. ERC-ESICM guidelines on temperature control after cardiac arrest in adults. Intensive Care Med. 2022;48(3):261–9.
Hassager C, Schmidt H, Møller JE, Grand J, Mølstrøm S, Beske RP, et al. Duration of device-based fever prevention after cardiac arrest. New Eng J Med. 2022;388:888–97
Jain A, Gray M, Slisz S, Haymore J, Badjatia N, Kulstad E. Shivering treatments for targeted temperature management: a review. J Neurosci Nurs. 2018;50(2):63–7.
Sessler DI. Thermoregulatory defense mechanisms. Crit Care Med. 2009;37(7 Suppl):S203–10.
Sessler DI. Defeating normal thermoregulatory defenses: induction of therapeutic hypothermia. Stroke. 2009;40(11):e614–21.
Oddo M, Frangos S, Maloney-Wilensky E, Andrew Kofke W, Le Roux PD, Levine JM. Effect of shivering on brain tissue oxygenation during induced normothermia in patients with severe brain injury. Neurocrit Care. 2010;12(1):10–6.
Madden LK, Hill M, May TL, Human T, Guanci MM, Jacobi J, et al. The implementation of targeted temperature management: an evidence-based guideline from the neurocritical care society. Neurocrit Care. 2017;27(3):468–87.
Choi HA, Ko SB, Presciutti M, Fernandez L, Carpenter AM, Lesch C, et al. Prevention of shivering during therapeutic temperature modulation: the Columbia anti-shivering protocol. Neurocrit Care. 2011;14(3):389–94.
Lenhardt R, Greif R, Sessler Daniel I, Laciny S, Rajek A, Bastanmehr H. Relative contribution of skin and core temperatures to vasoconstriction and shivering thresholds during isoflurane anesthesia. Anesthesiology. 1999;91(2):422–9.
Badjatia N, Strongilis E, Gordon E, Prescutti M, Fernandez L, Fernandez A, et al. Metabolic impact of shivering during therapeutic temperature modulation: the Bedside Shivering Assessment Scale. Stroke. 2008;39(12):3242–7.
Kasner SE, Wein T, Piriyawat P, Villar-Cordova CE, Chalela JA, Krieger DW, et al. Acetaminophen for altering body temperature in acute stroke: a randomized clinical trial. Stroke. 2002;33(1):130–4.
Polderman KH. Mechanisms of action, physiological effects, and complications of hypothermia. Crit Care Med. 2009;37(7 Suppl):S186-202.
Salinas P, Lopez-de-Sa E, Pena-Conde L, Viana-Tejedor A, Rey-Blas JR, Armada E, et al. Electrocardiographic changes during induced therapeutic hypothermia in comatose survivors after cardiac arrest. World J Cardiol. 2015;7(7):423–30.
Scirica BM. Therapeutic hypothermia after cardiac arrest. Circulation. 2013;127(2):244–50.
Polderman KH, Herold I. Therapeutic hypothermia and controlled normothermia in the intensive care unit: practical considerations, side effects, and cooling methods. Crit Care Med. 2009;37(3):1101–20.
Van Poucke S, Stevens K, Marcus AE, Lance M. Hypothermia: effects on platelet function and hemostasis. Thromb J. 2014;12(1):31.
Schefold JC, Storm C, Joerres A, Hasper D. Mild therapeutic hypothermia after cardiac arrest and the risk of bleeding in patients with acute myocardial infarction. Int J Cardiol. 2009;132(3):387–91.
Jacob M, Hassager C, Bro-Jeppesen J, Ostrowski SR, Thomsen JH, Wanscher M, et al. The effect of targeted temperature management on coagulation parameters and bleeding events after out-of-hospital cardiac arrest of presumed cardiac cause. Resuscitation. 2015;96:260–7.
Kimura A, Sakurada S, Ohkuni H, Todome Y, Kurata K. Moderate hypothermia delays proinflammatory cytokine production of human peripheral blood mononuclear cells. Crit Care Med. 2002;30(7):1499–502.
Mongardon N, Perbet S, Lemiale V, Dumas F, Poupet H, Charpentier J, et al. Infectious complications in out-of-hospital cardiac arrest patients in the therapeutic hypothermia era. Crit Care Med. 2011;39(6):1359–64.
Geurts M, Macleod MR, Kollmar R, Kremer PH, van der Worp HB. Therapeutic hypothermia and the risk of infection: a systematic review and meta-analysis. Crit Care Med. 2014;42(2):231–42.
Mongardon N, Legriel S, Lemiale V, Cariou A. Prediction of neurological outcome after cardiac arrest: is serum procalcitonin the future? Neurocrit Care. 2010;13(1):159–60.
Francois B, Cariou A, Clere-Jehl R, Dequin PF, Renon-Carron F, Daix T, et al. Prevention of early ventilator-associated pneumonia after cardiac arrest. N Engl J Med. 2019;381(19):1831–42.
Kirkegaard H, Grejs AM, Gudbjerg S, Duez C, Jeppesen A, Hassager C, et al. Electrolyte profiles with induced hypothermia: a sub study of a clinical trial evaluating the duration of hypothermia after cardiac arrest. Acta Anaesthesiol Scand. 2022;66(5):615–24.
Haase KK, Grelle JL, Khasawneh FA, Ike C. Variability in glycemic control with temperature transitions during therapeutic hypothermia. Crit Care Res Pract. 2017;2017:4831480.
Robba C, Siwicka-Gieroba D, Sikter A, Battaglini D, Dabrowski W, Schultz MJ, et al. Pathophysiology and clinical consequences of arterial blood gases and pH after cardiac arrest. Intensive Care Med Exp. 2020;8(Suppl 1):19.
Eastwood GM, Nielsen N, Nichol AD, Skrifvars MB, French C, Bellomo R. Reported practice of temperature adjustment (alpha-stat v pH-stat) for arterial blood gases measurement among investigators from two major cardiac arrest trials. Crit Care Resusc. 2019;21(1):69–71.
Empey PE, Miller TM, Philbrick AH, Melick JA, Kochanek PM, Poloyac SM. Mild hypothermia decreases fentanyl and midazolam steady-state clearance in a rat model of cardiac arrest. Crit Care Med. 2012;40(4):1221–8.
Iida Y, Nishi S, Asada A. Effect of mild therapeutic hypothermia on phenytoin pharmacokinetics. Ther Drug Monit. 2001;23(3):192–7.
Bjelland TW, Hjertner O, Klepstad P, Kaisen K, Dale O, Haugen BO. Antiplatelet effect of clopidogrel is reduced in patients treated with therapeutic hypothermia after cardiac arrest. Resuscitation. 2010;81(12):1627–31.
Sunjic KM, Webb AC, Sunjic I, Pala Creus M, Folse SL. Pharmacokinetic and other considerations for drug therapy during targeted temperature management. Crit Care Med. 2015;43(10):2228–38.
Sandroni C, D’Arrigo S, Nolan JP. Prognostication after cardiac arrest. Crit Care. 2018;22(1):150.
Kirkegaard H, Taccone FS, Skrifvars M, Soreide E. Postresuscitation care after out-of-hospital cardiac arrest: clinical update and focus on targeted temperature management. Anesthesiology. 2019;131(1):186–208.
Geocadin RG, Peberdy MA, Lazar RM. Poor survival after cardiac arrest resuscitation: a self-fulfilling prophecy or biologic destiny?*. Crit Care Med. 2012;40(3):979–80.
Hawkes MA, Rabinstein AA. Neurological prognostication after cardiac arrest in the era of target temperature management. Curr Neurol Neurosci Rep. 2019;19(2):10.
Geocadin RG, Callaway CW, Fink EL, Golan E, Greer DM, Ko NU, et al. Standards for studies of neurological prognostication in comatose survivors of cardiac arrest: a scientific statement from the American Heart Association. Circulation. 2019;140(9):e517–42.
Sandroni C, Geocadin RG. Neurological prognostication after cardiac arrest. Curr Opin Crit Care. 2015;21(3):209–14.
Elmer J, He Z, May T, Osborn E, Moberg R, Kemp S, et al. Precision care in cardiac arrest: ICECAP (PRECICECAP) study protocol and informatics approach. Neurocrit Care. 2022. https://doi.org/10.1007/s12028-022-01464-9.
Hong JM, Choi ES, Park SY. Selective brain cooling: a new horizon of neuroprotection. Front Neurol. 2022;13: 873165.
Zhou KQ, Davidson JO, Bennet L, Gunn AJ. Combination treatments with therapeutic hypothermia for hypoxic-ischemic neuroprotection. Dev Med Child Neurol. 2020;62(10):1131–7.
Acknowledgements
Funding
No funding or sponsorship was received for the publication of this article.
Author Contributions
Samantha Fernandez Hernandez, Brooke Barlow, Vera Pertsovskaya and Carolina B. Maciel contributed to the creation of this manuscript by drafting and editing the manuscript in its entirety.
Disclosures
Samantha Fernandez Hernandez, Brooke Barlow, Vera Pertsovskaya and Carolina B. Maciel have nothing to disclose.
Data availability
The data that support the findings of this scoping review are openly available in PubMed at https://pubmed.ncbi.nlm.nih.gov.
Compliance with Ethics Guidelines
This article is based on previously conducted studies and does not contain any new studies with human participants or animals performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Fernandez Hernandez, S., Barlow, B., Pertsovskaya, V. et al. Temperature Control After Cardiac Arrest: A Narrative Review. Adv Ther 40, 2097–2115 (2023). https://doi.org/10.1007/s12325-023-02494-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-023-02494-1