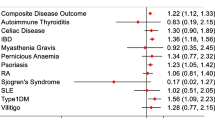
Abstract
Introduction
There are concerns that patients in an immunocompromised state may be at risk for increased coronavirus disease 2019 (COVID-19) severity. The aim of this study was to describe the characteristics of patients with COVID-19 and immune-mediated inflammatory diseases (IMIDs) or malignancies and evaluate their risk of developing severe COVID-19.
Methods
Cases of COVID-19 (ICD-10 code U07.1 or U07.2, or positive polymerase chain reaction or antigen test) among patients with IMIDs or malignancies were identified in the US-based Optum® Electronic Health Records database between 1 February 2020 and 3 March 2021. Age- and sex-standardized risks of severe COVID-19 were calculated by condition of interest. The risks were further adjusted by multiple covariates, and 95% confidence intervals were estimated.
Results
A total of 499,772 patients with COVID-19 were identified (mean [SD] age, 46.9 [20.7] years; 57.0% female). Patients with hematologic cancers (adjusted risk ratio [aRR] 2.0, 1.8–2.1), solid tumors (aRR 1.1, 1.1–1.1), or rheumatoid arthritis (aRR 1.2, 1.1–1.3) had a significantly higher risk of severe COVID-19 compared to the general population of patients with COVID-19. Patients with systemic lupus erythematosus (aRR 1.1, 0.9–1.2), psoriasis (aRR 1.0, 0.7–1.2), ulcerative colitis (aRR 0.9, 0.8–1.1), Crohn’s disease (aRR 0.9, 0.7–1.0), or ankylosing spondylitis (aRR 0.8, 0.5–1.0) showed a comparable risk of severe COVID-19. Patients with atopic dermatitis (aRR 0.8, 0.7–0.9) or psoriatic arthritis (aRR 0.8, 0.6–1.0) showed a lower risk of severe COVID-19.
Conclusions
The risk of developing severe COVID-19 varied between the studied IMIDs and malignancies. Patients with hematologic cancers, solid tumors, or rheumatoid arthritis had significantly increased risk for severe COVID-19 compared to the general population. These findings highlight the need to protect and monitor immunocompromised patients such as those with IMIDs or malignancies as part of the strategy to control the pandemic worldwide.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Why carry out this study? |
After COVID-19 developed into a pandemic with significant morbidity and mortality, both public health and healthcare professionals questioned the extent to which immunocompromising conditions, such as immune-mediated inflammatory diseases (IMIDs) or malignancies, increased patients’ risk of SARS-CoV-2 infections and related adverse outcomes. |
While patients in immunocompromised states are generally considered to be at risk for severe COVID-19, the risk has not been evaluated across major IMIDs and malignancies to understand how each individual disease contributes to COVID-19 outcomes using the same data source. |
What was learned from the study? |
The risk of developing severe COVID-19 varied among each studied IMID and the types of malignancies. Patients with rheumatoid arthritis were at a higher risk than those with other IMIDs for severe COVID-19 after adjusting for demographic and clinical characteristics. Notably, patients with rheumatoid arthritis were significantly older and had a higher Charlson comorbidity index score than the general population of patients with COVID-19. |
While it is important to protect all patients with IMIDs or malignancies from exposure to SARS-CoV-2, COVID-19 monitoring and management should be pertinent for each IMID or type of malignancies in addition to other risk factors for severe COVID-19. |
Introduction
The coronavirus disease 2019 (COVID-19) has become a disastrous pandemic since severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was first detected in December 2019 [1]. As of March 2022, more than 460 million people across nearly 200 countries were infected [1, 2], and approximately 6 million COVID-19-associated deaths were reported worldwide [2]. In the USA, the total confirmed cases reached over 79.3 million, and roughly 965,336 deaths were attributed to COVID-19, placing COVID-19 as the third leading cause of death in the USA in 2020 [3].
Immunocompromised patients, such as those with immune-mediated inflammatory diseases (IMIDs) or malignancies, may be at higher risk for severe outcomes from COVID-19 because of an underlying weakened immune system and/or immunosuppression [4]. In particular, patients with malignancies or IMIDs have a weakened immune status, often have multiple underlying comorbidities, take immunosuppressive medicines, and are more susceptible to infections [5,6,7,8,9,10]. While several studies have evaluated the risk of severe COVID-19 in patients with malignancies or IMIDs, there is still a lack of understanding of how the risks differ among individual conditions [11,12,13,14]. Earlier studies were often limited by small sample sizes during the early period of the pandemic. As a result, those studies were unable to thoroughly evaluate and compare the outcomes of COVID-19 among patients with individual IMIDs and types of malignancies [15,16,17].
As the COVID-19 pandemic continues, understanding the clinical outcomes of patients with IMIDs or malignancies has become critically important. Utilizing a US nationwide electronic health records (EHR) database collected from routine clinical practices across a national network of healthcare providers, this study aimed to evaluate and compare the risk of severe COVID-19 among patients with selected IMIDs and malignancies. In this study, the evaluated conditions of interest included the individual IMIDs of ankylosing spondylitis (AS), atopic dermatitis (AD), psoriasis (PsO), psoriatic arthritis (PsA), rheumatoid arthritis (RA), Crohn’s disease (CD), ulcerative colitis (UC), systemic lupus erythematosus (SLE), and subgroups of malignancies of solid tumors (ST) and hematologic cancers (HC).
Methods
Data Source
The Optum® de-identified Electronic Health Records (Optum® EHR) database contains longitudinal patient care data from healthcare provider organizations with more than 700 hospitals and 7000 clinics across the USA and captures point-of-care diagnostic data that are specific to COVID-19, including patient-level and clinical results from both inpatient and ambulatory settings [18]. All 50 states in the USA and all types of payors are represented, including Medicare, Medicaid, commercial insurers, and self-pay/uninsured. The Optum® EHR provides near-real-time data on the COVID-19 pandemic. Regardless of the test result, patients with a history of any COVID-19 test or diagnosis are included in the Optum® EHR database. All pertinent available data on patients within the cohort are provided back to 2007, and new patients are added to the cohort at each data refresh. The data include patient demographics, inpatient and outpatient visits, comprehensive laboratory data, medications prescribed and administered, and procedures.
The Optum® EHR database included records for: conducted polymerase chain reaction (PCR) and antigen testing for SARS-CoV-2; the results of the SARS-CoV-2 testing, which were verified by a team of medical terminologists and clinicians via manual review; and clinical diagnosis for COVID-19 and other coronavirus-related infections, which can be used to identify patients with SARS-CoV-2 infection. The Optum® EHR database is certified as de-identified following the Health Insurance Portability and Accountability Act’s (HIPAA) statistical de-identification rules. The database is managed according to the Optum® customer data use agreements and therefore this study was exempt from institutional review board (IRB) approval.
Study Population
Patients with COVID-19 were identified using International Statistical Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM) diagnosis codes (U07.1 and U07.2), which were introduced on 1 April 2020, or with a positive laboratory diagnostic test result by a SARS-Cov-2 virus PCR or antigen test [19]. The COVID-19 diagnosis date was defined as the earliest of any positive PCR or antigen test, or diagnosis for COVID-19. The COVID-19 diagnosis dates ranged between 1 February 2020 and 3 March 2021, and the last follow-up date was 28 April 2021.
A minimum of 15 months of active EHRs prior to the index date was required to allow sufficient time to capture patients’ baseline demographics and clinical characteristics. Patients with missing age or sex were excluded. Within this study cohort of all eligible patients with SARS-CoV-2 infection, we created ten subgroups based on their diagnosis for the following conditions of interest within 15 months before the index date of COVID-19 diagnosis: RA, PsA, AS, SLE, PsO, AD, CD, UC, ST, and HC. Only one diagnosis code (in any setting and position) was required, and the conditions of interest were not mutually exclusive. Patients were followed for 3 months from the index date, or until a censoring event, which was defined as death, end of active EHR, or end of data availability (28 April 2021), whichever was earliest.
Study Variables
Demographic characteristics included patient age group (0–17, 18–44, 45–64, 65–74, 75–84, 85+ years), sex, race, ethnicity, geographic region (Midwest, Northeast, South, and West), and calendar quarter (Jan–Mar 2020, Apr–Jun 2020, Jul–Sept 2020, Jan–Mar 2021) of SARS-CoV-2 infection measured as the index date. We identified the following seven underlying at-risk medical conditions, adapted from the Centers for Disease Control and Prevention (CDC) 2019 Novel Coronavirus case report form [20]: cardiovascular disease (CVD), chronic kidney disease, chronic liver disease, chronic lung disease, malignancy, diabetes, and obesity (body mass index [BMI] ≥ 30). Charlson comorbidity index (CCI) score, and underlying at-risk medical conditions were captured during the 15 months prior to the index date (Fig. 1) [21, 22].
Outcomes
The primary outcome was severe COVID-19, which was defined as either hospitalization within the 14 days prior to and 30 days following the COVID-19 diagnosis, or death within 3 months of the COVID-19 diagnosis. Hospitalization and death were analyzed separately as secondary outcomes. Death data were obtained from the Social Security Administration Death Master File, which reports month and year of death but does not report the date; in order to calculate the number of days between diagnosis and death, the date of death was set as the 15th of the month. Hospitalization within the 14 days prior to the COVID-19 diagnosis was also identified because (1) date of death is assigned on the 15th of the month of death and therefore using 14 days prior to diagnosis will balance out misclassification in both directions assuming an uniform distribution; and (2) because of the shortage of testing during the earlier phase of COVID-19 pandemic, many positive test results came back after hospitalization and death. Therefore, including 14 days prior ensured a more complete capture of COVID-19 outcomes.
Statistical Analysis
Patient demographics and clinical characteristics were summarized with descriptive statistics. Mean and standard deviation (SD) were reported for continuous variables, and count (N) and proportion (%) were reported for categorical variables. Age- and sex-standardized risk of death, hospitalization, and severe COVID-19 were calculated for comparison and extrapolation using direct standardization with the 2010 US Census data as the standard population [23]. For each condition of interest, imbalances of covariates with respect to the general population of patients with COVID-19 were adjusted by inverse probability weighting (IPW), and confidence intervals (CI) were calculated by bootstrapping. Adjusted risk ratios (aRR) and 95% CIs were estimated. The covariates used for adjustments included baseline demographics, clinical characteristics, and at-risk underlying conditions. The standardized mean differences (SMDs) between all patients with COVID-19 and each condition of interest were calculated to measure the imbalance between patient groups and the general cohort of patients with COVID-19. An SMD < 0.2 was considered an inconsequential imbalance. The descriptive analysis was conducted using SAS Studio 3.81 (SAS Institute Inc., Cary, NC, USA), and the rest of the analyses were performed using R Studio version 3.6.0 (R Studio, Boston, MA, USA) and R packages generalize and tableone.
Results
Baseline Patient Demographics and Clinical Characteristics
Patients with SARS-CoV-2 Infection
We identified 499,772 eligible patients with SARS-CoV-2 infection between 1 February 2020 and 3 March 2021 (Fig. 2). Overall, the mean (SD) age at cohort entry was 46.9 (SD 20.7) years, with 57.0% female and 72.5% Caucasian. The majority of the SARS-CoV-2 infections were confirmed with laboratory testing by positive PCR (349,055 [69.9%]) or positive antigen test (15,125 [3.0%]), while the remaining cases (27%) were identified solely on the basis of recorded clinical diagnosis (Fig. 3). Vaccination status appeared to be under-captured in this EHR database. Through all available data (28 April 2021), only 28,926 (5.8%) of patients had records for receiving at least one dose of a COVID-19 vaccine while the national coverage was estimated at 57% in May 2021 [24].
Many conditions of interest represented a small proportion of the general cohort of patients with COVID-19, often less than 1%. Patients diagnosed with ST were the largest group, consisting of 42,126 patients (8.4% of the general cohort of patients with COVID-19). In general, patients with any condition of interest tended to be older than the general cohort of patients with COVID-19, while patients with AD (mean age of 34.9 years) were younger than the general cohort. Other patient demographics and clinical characteristics varied between conditions of interest as well (Table 1).
Patients with Severe COVID-19 Outcomes
Among the 499,772 patients with COVID-19 in the study, 67,584 patients (13.5%) developed severe COVID-19 after SARS-CoV-2 infection, including 63,125 hospitalizations and 14,177 deaths. As these were not mutually exclusive events, of the 63,125 hospitalized patients, 9718 (15.4%) died within 3 months from the index date, which accounted for 68.6% of the 14,177 deaths during the follow-up period.
Patients with and without severe COVID-19 outcomes (hospitalization and/or death) had different patient profiles (Table 2). Patients with severe COVID-19 were older (mean age of 63.5 years vs of 44.3 years, SMD = 1.01). They had different demographic backgrounds in race, geographic region, and varied in their calendar time of COVID-19 diagnosis/positive test, although sex distribution was comparable. Baseline health status estimated by CCI score was worse for patients who had severe COVID-19 outcomes when compared to the CCI among patients without severe COVID-19.
Patients with Underlying At-Risk Conditions
Of all patients with COVID-19, 280,302 (56.1%) had one or more underlying at-risk medical conditions prior to the index date, with CVD as the most common condition (34.9%), closely followed by obesity (34.4%, Fig. 4). Compared with the general cohort of patients with COVID-19, a higher proportion of patients with a condition of interest had underlying at-risk medical conditions. Having four or more comorbid at-risk conditions was more common in all conditions of interest when compared to the general COVID-19 cohort, ranging from 7.3% to 34.9%, compared to 6.2% in the general COVID-19 cohort (Fig. 5).
Frequency (%) of underlying medical conditions among patients diagnosed with COVID-19, by condition of interest. All general population of patients with COVID-19, RA rheumatoid arthritis, PsA psoriatic arthritis, AS ankylosing spondylitis, SLE systemic lupus erythematosus, PsO psoriasis, AD atopic dermatitis, CD Crohn’s disease, UC ulcerative colitis, ST solid tumor, HC hematologic cancers, CVD cardiovascular disease, BMI body mass index
Distribution of total underlying medical conditions among patients diagnosed with COVID-19, by condition of interest. All general population of patients with COVID-19, RA rheumatoid arthritis, PsA psoriatic arthritis, AS ankylosing spondylitis, SLE systemic lupus erythematosus, PsO psoriasis, AD atopic dermatitis, CD Crohn’s disease, UC ulcerative colitis, ST solid tumor, HC hematologic cancers
Severe COVID-19 Outcomes
With age and sex standardization to the 2010 US Census data, the risk of severe COVID-19 among the general cohort of patients with COVID-19 was 9.7%, with a hospitalization risk of 9.2% and mortality risk of 1.7%. By condition of interest, the age- and sex-standardized risk of severe COVID-19 outcomes varied: patients with HC (21.5%), SLE (18.1%), ST (13.1%), and RA (12.1%) had a higher risk than the general COVID-19 cohort; patients with PsA (7.4%), AS (7.2%), and AD (7.0%) had a lower risk than the general cohort; patients with UC (9.5%), CD (9.5%), and PsO (9.1%) had a comparable risk to the general cohort (Fig. 6).
Age- and sex-standardized risk of severe COVID-19 outcomes among patients diagnosed with COVID-19, by condition of interest. All general population of patients with COVID-19, RA rheumatoid arthritis, PsA psoriatic arthritis, AS ankylosing spondylitis, SLE systemic lupus erythematosus, PsO psoriasis, AD atopic dermatitis, CD Crohn’s disease, UC ulcerative colitis, ST solid tumors, HC hematologic cancers
We compared baseline characteristics between patients with each condition of interest and the general COVID-19 cohort. Outcomes were adjusted for demographic factors (i.e., age, sex, race, ethnicity, geographic region) and potential COVID-19 risk factors, including CCI score, BMI, diabetes, CVD, and chronic lung disease. After adjustments, age was still not comparable for patients with RA, SLE, PsA, and HC when compared to the general COVID-19 cohort (Fig. 7). Additionally, BMI and CCI score often showed moderate differences in its distribution between patients with a condition of interest compared to the general COVID-19 cohort.
Standardized mean difference between disease condition and all patients with COVID-19 before and after adjustment. CCI Charlson comorbidity index, BMI body mass index. The adjusted covariates were with age, gender, race, ethnicity, BMI, region of residence, CCI, diabetes, chronic lung disease, and cardiovascular disease. The dashed line represents SMD = 0.2
The risk of severe COVID-19 outcomes varied by condition of interest. Compared to the general COVID-19 cohort, patients with HC (aRR 2.0, 1.8–2.1), RA (aRR 1.2, 1.1–1.3), and ST (aRR 1.1, 1.1–1.1) were estimated to be at a higher risk of severe COVID-19. In contrast, the patients with PsA (aRR 0.8, 0.6–1.0) or AD (aRR 0.8, 0.7–0.9) had a lower risk of severe COVID-19 compared to the general population with COVID-19. Patients with SLE (aRR 1.1, 0.9–1.2), PsO (aRR 1.0, 0.7–1.2), UC (aRR 0.9, 0.8–1.1), CD (aRR 0.9, 0.7–1.0), and AS (aRR 0.8, 0.5–1.0) showed a comparable risk of severe COVID-19 (Fig. 8 and Supplementary Table S1). Separate analyses were conducted for hospitalization and death. With the exception of CD and AS, the risks of hospitalization and death were similar to the risk of severe COVID-19 (Supplementary Fig. S1 and Table S1).
Discussion
This study utilized a nationwide EHR database in the USA to examine patient demographics, clinical characteristics, and severe COVID-19 in a large cohort of patients with COVID-19 between February 2020 and March 2021. This study examined major IMIDs and malignancies of interest separately and then compared them to the general population of patients with COVID-19 within a large cohort in the USA.
We found that patients with RA, ST, or HC were at significantly increased risk for COVID-19-related hospitalization, death, and severe COVID-19 compared to general patients with COVID-19 after statistical adjustments. These findings are consistent with previous studies among patients with RA [25,26,27] and patients with malignancies [11, 28]. However, a large cohort study using the US TriNetX data found that the risk of severe COVID-19, including death and hospitalization, was not significantly different between patients with and without RA [29]. Patients with HC were at a higher risk than those with ST for severe COVID-19 outcomes. In a retrospective case–control study, Wang et al. reported that patients with malignancies and COVID-19 had significantly worse outcomes, especially for patients with HC, such as leukemia or non-Hodgkin’s lymphoma [11].
We also found that patients with UC or CD were not at higher risk for severe COVID-19 than the general population with COVID-19. These findings are similar to previous reports for patients with inflammatory bowel disease (IBD) in Italy and Sweden [30, 31]. Moreover, a multicenter research network study found that patients with IBD and COVID-19 were not at an increased risk for hospitalization or COVID-19-related death compared to patients without IBD (relative risk 0.93; 95% CI 0.68–1.27) [32]. We observed that patients with AD showed a lower risk of being hospitalized or developing severe COVID-19, even after adjusting for age. Current evidence indicates that patients with AD are not at increased risk of hospitalization or death [33, 34]. Keswani et al. found that AD was inversely associated with COVID-19 hospitalization [35]. These results, including the widely different risk ratios for individual conditions, indicate that the risk of severe COVID-19 may vary across different IMIDs. Our study showed that the risk for COVID-19 hospitalization in patients with PsA was lower than that in patients with other IMIDs (e.g., RA, SLE, UC or CD). Similarly, Curtis et al. found that patients with RA or UC had a higher incidence of hospitalization than patients with PsA [36].
After IPW adjustment, more standardized differences for covariates reducing below 0.2 shows that the adjustment attenuated the extent of variability in patient demographics and baseline characteristics between the patients with conditions of interest and the general cohort of patients with COVID-19. However, considerable differences in baseline demographics and patient characteristics still existed. This might reflect the nature of the patient population rather than true bias. For example, the AD population with COVID-19 was noticeably younger than the general COVID-19 population, due to the early age of onset for AD [37]. SLE is more common in young women [38,39,40], which was reflected in the large imbalance in gender distribution in our lupus population with COVID-19. Malignancy generally affects senior populations more than younger ones, and therefore we were not surprised to see the higher mean age among patients with ST and HC who contracted COVID-19. When assessing patient risk, a comprehensive patient profile, including patients’ demographics and other underlying medical conditions, must be considered to make sure that patient outcomes are comparable.
Consistent with existing literature of risk factors associated with severe COVID-19, older age [41, 42], underlying CVD [39, 42], diabetes [42, 43], obesity [39, 44], and high CCI score [45] were more common among patients with conditions of interest experiencing severe COVID-19 than those patients who did not. Differences in COVID-19 diagnosis index periods between those with and without severe COVID-19 might reflect the improvement in patient management during the latter period of the pandemic, and/or potentially associated with the availability of COVID-19 vaccines and diagnostic tests. Vaccinated patients with COVID-19 are less likely to require hospitalization and have disease progression to mechanical ventilation or death compared with unvaccinated patients [46, 47].
A few limitations of this study should be noted as well. First of all, hospitalizations and care external to the healthcare delivery network contributing to the EHR data source may not be captured, and centers contributing to the Optum EHR data may not be fully representative of the general population in the USA. We do not believe this limitation would appear across our conditions of interest differently; therefore, while this limitation might affect the absolute risk assessment for the study outcomes, the impact for the relative risk assessment for severe COVID-19 between conditions of interest and the general population was minimal. Second, COVID-19 vaccination status was not evaluated in this study. It might have influenced the number of patients included in the cohort and their outcomes from its availability in mid-December 2020 when the US Food and Drug Administration (FDA) approved COVID-19 vaccines with emergency use authorization in the USA but the influence might not be substantial given the extremely limited vaccine access the general population had during the study period [48]. As a reference, by 3 March 2021 (i.e., last cohort entry date in this study), a small proportion of 9% (n = 32.5 million) of the US population were fully vaccinated [49, 50]. Because COVID-19 vaccines were not broadly available to the general US population during the study period, COVID-19 vaccine data were extremely under-captured and potentially unreliable in this EHR data source. Many patients received their first COVID-19 vaccine doses at community pharmacies or mass vaccination sites outside of hospitals or clinics. This required patients to self-report vaccine status to their medical care providers. Additionally, due to limitations in data availability, including 5.7% of the study population having less than 3 months of follow-up, death was only assessed during the 3 months after the index date, which may lead to an underestimation. Serious long-term consequences from SARS-CoV-2 infection are still not fully understood [51]. A better understanding of the long-term consequences and implications of COVID-19 in patients with IMIDs or malignancies is urgently needed to guide clinicians in the care of these patients. Lastly, this study was subject to residual confounding from factors such as variants of SARS-CoV-2 and disease severity which was not available in the data source. Another source of potential confounding was the immunosuppressive therapies which may increase the risk and severity of COVID-19 for patients with a condition of interest. The extent of an individual’s immunocompromised state may differ by both disease severity and activity. This also could be potentially further complicated by treatment regimens, as many therapeutic options for IMIDs and malignancies contribute to immunosuppression. However, a few studies reported no association between immunosuppressive treatment (e.g., disease-modifying antirheumatic drugs) and severe COVID-19 outcomes [52,53,54].
One strength of this study is the level of granularity it adds to the current literature on evaluating the risk of severe COVID-19 among patients with major IMIDs by individual condition within a large nationwide cohort. In the current literature, often only a few of these conditions of interest are assessed, often specific conditions are not compared against each other or are compiled together to compare against a general population, since an estimated prevalence of IMIDs collectively ranges between 5% and 10% in the industrial population [29, 53, 55, 56]. The results of this study suggest the risk of severe COVID-19 varies by condition within IMIDs. In addition, the use of large-scale, nationwide COVID-19 data in this study allowed for a more comprehensive assessment of patients diagnosed with COVID-19. In addition to the available COVID-19 diagnosis codes, diagnostic test results were professionally reviewed by the data provider for their reliability to identify positive results. This may have captured additional patients with milder infections who may have not utilized further COVID-19-related medical services. EHR as a data source is payer agnostic, and it is less influenced by changes in eligible patients from societal consequences with COVID-19 epidemics, such as loss of employer-based health insurance and transition to government-sponsored coverage (e.g., Medicaid, Medicare) or to individual market coverage [57].
Conclusion
Using a nationwide EHR database, this study evaluated whether patients with underlying IMIDs or malignancies were more vulnerable to severe COVID-19 outcomes because of the immunosuppressive nature of such conditions. The risk of severe COVID-19 varied by condition. Compared to the general population of all patients diagnosed with COVID-19, patients with RA, ST, and HC were at a higher risk of severe COVID-19 outcomes (i.e., hospitalization or death). These findings highlight the need to protect and monitor patients with specific immunocompromising diseases as part of the strategy to mitigate risks for severe COVID-19 in this ongoing pandemic.
References
WHO Director-General’s remarks at the media briefing on 2019-nCoV on 11 February 2020. https://www.who.int/dg/speeches/detail/who-director-general-s-remarks-at-the-media-briefing-on-2019-ncov-on-11-february-2020.
WHO coronavirus disease (COVID-19) Dashboard. https://covid19.who.int/.
United States COVID-19 cases, deaths, and laboratory testing (NAATs) by state, territory, and jurisdiction. https://covid.cdc.gov/covid-data-tracker/#cases-deaths-testing-trends. Accessed 8 Mar 2022.
Woolf SH, Chapman DA, Lee JH. COVID-19 as the leading cause of death in the United States. JAMA. 2021;325(2):123–4.
Kitsis RN, Riquelme JA, Lavandero S. Heart disease and cancer: are the two killers colluding? Circulation. 2018;138:692–5.
Giovannucci E, Harlan DM, Archer MC, et al. Diabetes and cancer: a consensus report. CA: Cancer J Clin. 2010;60(4):207–21.
Ogle KS, Swanson GM, Woods N, Azzouz F. Cancer and comorbidity: redefining chronic diseases. Cancer. 2000;88(3):653–63.
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331(6024):1565–70.
Tan EH, Sena AG, Prats-Uribe A, et al. COVID-19 in patients with autoimmune diseases: characteristics and outcomes in a multinational network of cohorts across three countries. Rheumatology. 2021. https://doi.org/10.1093/rheumatology/keab250.
Triolo TM, Armstrong TK, McFann K, et al. Additional autoimmune disease found in 33% of patients at type 1 diabetes onset. Diabetes Care. 2011;34(5):1211–3.
Wang Q, Berger NA, Xu R. Analyses of risk, racial disparity, and outcomes among US patients with cancer and COVID-19 infection. JAMA Oncol. 2021;7(2):220–7.
D’Silva KM, Serling-Boyd N, Wallwork R, et al. Clinical characteristics and outcomes of patients with coronavirus disease 2019 (COVID-19) and rheumatic disease: a comparative cohort study from a US ‘hot spot.’ Ann Rheum Dis. 2020;79(9):1156–62.
Ungaro RC, Agrawal M, Park S, et al. Autoimmune and chronic inflammatory disease patients with COVID-19. ACR Open Rheumatol. 2021;3(2):111–5.
MacKenna B, Kennedy NA, Mehrkar A, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. Lancet Rheumatol. 2022;4(7):e490–e506.
Faye AS, Lee KE, Laszkowska M, et al. Risk of adverse outcomes in hospitalized patients with autoimmune disease and COVID-19: a matched cohort study from New York City. J Rheumatol. 2021;48(3):454–62.
Kuderer NM, Choueiri TK, Shah DP, et al. Clinical impact of COVID-19 on patients with cancer (CCC19): a cohort study. Lancet. 2020;395(10241):1907–18.
Liu M, Gao Y, Zhang Y, Shi S, Chen Y, Tian J. The association between severe or dead COVID-19 and autoimmune diseases: a systematic review and meta-analysis. J Infect. 2020;81(3):e93–5.
Optum, Inc. Optum EHR data. https://www.optum.com/business/solutions/life-sciences/real-world-data/ehr-data.html.
Centers for Disease Control and Prevention. ICD-10-CM official coding and reporting guidelines: April 1, 2020 through September 30, 2020. https://www.cdc.gov/nchs/icd/icd10cm.htm.
Human infection with 2019 novel coronavirus case report form. https://www.cdc.gov/coronavirus/2019-ncov/downloads/pui-form.pdf.
Thygesen SK, Christiansen CF, Christensen S, Lash TL, Sørensen HT. The predictive value of ICD-10 diagnostic coding used to assess Charlson comorbidity index conditions in the population-based Danish National Registry of Patients. BMC Med Res Methodol. 2011;11(1):1–6.
Glasheen WP, Cordier T, Gumpina R, Haugh G, Davis J, Renda A. Charlson comorbidity index: ICD-9 update and ICD-10 translation. Am Health Drug Benefits. 2019;12(4):188.
The U.S. Census Bureau. Age and Sex Composition in the United States: 2010. https://www.census.gov/data/tables/2010/demo/age-and-sex/2010-age-sex-composition.html.
Diesel J, Sterrett N, Dasgupta S, et al. COVID-19 vaccination coverage among adults—United States, December 14, 2020–May 22, 2021. Morb Mortal Wkly Rep. 2021;70(25):922.
Hyrich KL, Machado PM. Rheumatic disease and COVID-19: epidemiology and outcomes. Nat Rev Rheumatol. 2021;17(2):71–2.
England BR, Roul P, Yang Y, et al. Risk of COVID-19 in rheumatoid arthritis: a national veterans affairs matched cohort study in at-risk individuals. Arthritis Rheumatol. 2021;73(12):2179–88.
Haberman R, Axelrad J, Chen A, et al. Covid-19 in immune-mediated inflammatory diseases—case series from New York. N Engl J Med. 2020;383(1):85–8.
Liang W, Guan W, Chen R, et al. Cancer patients in SARS-CoV-2 infection: a nationwide analysis in China. Lancet Oncol. 2020;21(3):335–7.
Raiker R, DeYoung C, Pakhchanian H, et al. Outcomes of COVID-19 in patients with rheumatoid arthritis: a multicenter research network study in the United States. Semin Arthritis Rheum. 2021;51:1057–66.
Bezzio C, Saibeni S, Variola A, et al. Outcomes of COVID-19 in 79 patients with IBD in Italy: an IG-IBD study. Gut. 2020;69(7):1213–7.
Ludvigsson JF, Axelrad J, Halfvarson J, et al. Inflammatory bowel disease and risk of severe COVID-19: a nationwide population-based cohort study in Sweden. UEG J. 2021;9(2):177–92.
Singh S, Khan A, Chowdhry M, Bilal M, Kochhar GS, Clarke K. Risk of severe coronavirus disease 2019 in patients with inflammatory bowel disease in the United States: a multicenter research network study. Gastroenterology. 2020;159(4):1575-1578. e1574.
Nguyen C, Yale K, Casale F, et al. SARS-CoV-2 infection in patients with atopic dermatitis: a cross-sectional study. Br J Dermatol. 2021;185(3):640–1.
Yang JM, Koh HY, Moon SY, et al. Allergic disorders and susceptibility to and severity of COVID-19: a nationwide cohort study. J Allergy Clin Immunol. 2020;146(4):790–8.
Keswani A, Dhana K, Rosenthal JA, Moore D, Mahdavinia M. Atopy is predictive of a decreased need for hospitalization for coronavirus disease 2019. Ann Allergy Asthma Immunol. 2020;125(4):479.
Curtis JR, Zhou X, Rubin DT, et al. Characteristics, comorbidities, and outcomes of SARS-CoV-2 infection in patients with autoimmune conditions treated with systemic therapies: a population-based study. J Rheumatol. 2022;49(3):320–9.
Williams HC. Epidemiology of atopic dermatitis. Clin Exp Dermatol. 2000;25(7):522–9.
Moroni L, Bianchi I, Lleo A. Geoepidemiology, gender and autoimmune disease. Autoimmun Rev. 2012;11(6):A386–92.
Booth A, Reed AB, Ponzo S, et al. Population risk factors for severe disease and mortality in COVID-19: a global systematic review and meta-analysis. PLoS ONE. 2021;16(3): e0247461.
Barber MR, Drenkard C, Falasinnu T, et al. Global epidemiology of systemic lupus erythematosus. Nat Rev Rheumatol. 2021;17(9):515–32.
Harrison SL, Fazio-Eynullayeva E, Lane DA, Underhill P, Lip GY. Comorbidities associated with mortality in 31,461 adults with COVID-19 in the United States: a federated electronic medical record analysis. PLoS Med. 2020;17(9): e1003321.
Zheng Z, Peng F, Xu B, et al. Risk factors of critical & mortal COVID-19 cases: a systematic literature review and meta-analysis. J Infect. 2020;81(2):e16–25.
Ko JY, Danielson ML, Town M, et al. Risk factors for COVID-19-associated hospitalization: COVID-19-associated hospitalization surveillance network and behavioral risk factor surveillance system. Clin Infect Dis. 2021;72(11):e695–e703.
Cunningham JW, Vaduganathan M, Claggett BL, et al. Clinical outcomes in young US adults hospitalized with COVID-19. JAMA Intern Med. 2021;181(3):379–81.
Kuswardhani RT, Henrina J, Pranata R, Lim MA, Lawrensia S, Suastika K. Charlson comorbidity index and a composite of poor outcomes in COVID-19 patients: a systematic review and meta-analysis. Diabetes Metab Syndr. 2020;14(6):2103–9.
Tenforde MW, Self WH, Adams K, et al. Association between mRNA vaccination and COVID-19 hospitalization and disease severity. JAMA. 2021;326(20):2043–54.
Klompas M. Understanding breakthrough infections following mRNA SARS-CoV-2 vaccination. JAMA. 2021;326(20):2018–20.
FDA takes additional action in fight against COVID-19 by issuing Emergency Use Authorization for second COVID-19 vaccine. https://www.fda.gov/news-events/press-announcements/fda-takes-additional-action-fight-against-covid-19-issuing-emergency-use-authorization-second-covid.
COVID-19 Vaccinations in the United States, Jurisdiction. https://data.cdc.gov/Vaccinations/COVID-19-Vaccinations-in-the-United-States-Jurisdi/unsk-b7fc.
Centers for Disease Control and Prevention. Cumulative count of fully vaccinated people reported to CDC by date administered, United States. https://covid.cdc.gov/covid-data-tracker/#vaccination-trends_vacctrends-fully-cum.
Mainous AG 3rd, Rooks BJ, Wu V, Orlando FA. COVID-19 post-acute sequelae among adults: 12 month mortality risk. Front Med. 2021;2351. https://doi.org/10.3389/fmed.2021.778434.
Pablos JL, Galindo M, Carmona L, et al. Clinical outcomes of hospitalised patients with COVID-19 and chronic inflammatory and autoimmune rheumatic diseases: a multicentric matched cohort study. Ann Rheum Dis. 2020;79(12):1544–9.
Gianfrancesco M, Hyrich KL, Al-Adely S, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Ann Rheum Dis. 2020;79(7):859–66.
Ye Y, Yue X, Krueger W, Wegrzyn L. POS1163 characteristics and outcomes in a real-world cohort of rheumatoid arthritis patients with COVID-19. Ann Rheum Dis. 2021. https://doi.org/10.1136/annrheumdis-2021-eular.565.
Shapira Y, Agmon-Levin N, Shoenfeld Y. Geoepidemiology of autoimmune rheumatic diseases. Nat Rev Rheumatol. 2010;6(8):468–76.
Shoenfeld Y, Selmi C, Zimlichman E, Gershwin ME. The autoimmunologist: geoepidemiology, a new center of gravity, and prime time for autoimmunity. J Autoimmun. 2008;31(4):325–30.
McDermott D, Cox C, Rudowitz R, Garfield R. How has the pandemic affected health coverage in the U.S.? San Francisco: Kaiser Family Foundation; 2020.
Acknowledgements
Funding
AbbVie provided financial support for the study including associated publication costs such as the journal’s Rapid Service and Open Access Fees. AbbVie participated in the study design, research, data collection, analysis and interpretation of data, writing, reviewing, and approving the publication. All authors had access to the data results, and participated in the development, review, and approval of this abstract. No honoraria or payments were made for authorship.
Editorial Assistance
Editorial assistance was provided by Dewilka Simons from AbbVie.
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author Contributions
Xiaomeng Yue, Yizhou Ye, Yookyung C Choi, Dongmu Zhang, and Whitney S Krueger contributed to the conception and design of the study, the interpretation of data, critical revision of the manuscript, agree to be accountable for ensuring the integrity and accuracy of the work, and approved the final version of the manuscript.
Disclosures
Xiaomeng Yue, Yizhou Ye, Yookyung C Choi, Dongmu Zhang, and Whitney S Krueger are employees of AbbVie Inc. and may own AbbVie stock or options.
Compliance with Ethics Guidelines
The authors affirm that this retrospective study using a de-identified data source did not entail collection, use, or transmittal of identifiable data. Based on 45CFR46.101(b)(4): Existing Data & Specimens—No Identifiers, this study was exempt from the requirement for institutional review board approval. The study was compliant with data security requirements of the Health Insurance Portability and Accountability Act of 1996.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Author information
Authors and Affiliations
Corresponding author
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License, which permits any non-commercial use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by-nc/4.0/.
About this article
Cite this article
Yue, X., Ye, Y., Choi, Y.C. et al. Risk of Severe COVID-19 Outcomes Among Patients with Immune-Mediated Inflammatory Diseases or Malignancies: A Retrospective Analysis of Real-World Data in the United States. Adv Ther 39, 5413–5432 (2022). https://doi.org/10.1007/s12325-022-02293-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-022-02293-0