Abstract
Introduction
Telmisartan, rosuvastatin and ezetimibe are commonly recommended as combination therapies. However, the pharmacokinetic (PK) interaction among these therapeutic drugs has not been clearly reported. The objective of this study was to investigate possible interactions between telmisartan monotherapy and a fixed-dose combination (FDC) of rosuvastatin/ezetimibe.
Methods
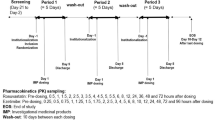
A randomized, open-label, multiple oral dose, three-treatment, three-period, six-sequence crossover study was conducted in healthy male volunteers. Monotherapy and cotherapy with telmisartan (80 mg) or a FDC of rosuvastatin and ezetimibe (20/10 mg) were compared after once-daily treatment for 7 days. The PK profiles for telmisartan, rosuvastatin, total ezetimibe (ezetimibe + exetimibe glucuronide) and ezetimibe were evaluated up to 48 h after the last dose. There was a 14-day washout period between each treatment.
Results
The geometric mean ratios (GMRs) and 90% confidence intervals (CIs) for the peak plasma concentration at steady state (Cmax,ss) and area under the plasma concentration-time curve during the dosing interval at steady state (AUCτ,ss) were 1.258 (1.072–1.475) (P = 0.020) and 1.264 (1.167–1.370) (P < 0.001) for telmisartan, 0.796 (0.723–0.878) (P < 0.001) and 0.904 (0.842–0.970) (P = 0.021) for total ezetimibe and 1.237 (1.081–1.416) (P = 0.012) and 0.988 (0.899–1.086) (P = 0.832) for ezetimibe, respectively. With rosuvastatin, the GMR (90% CI) was 2.616 (2.287–2.992) (P < 0.001) for Cmax,ss and 1.265 (1.168–1.369) (P < 0.001) for AUCτ,ss. No serious adverse events or clinically significant results were reported.
Conclusions
The coadministration of multiple doses of telmisartan and rosuvastatin/ezetimibe led to a mild increase in systemic exposure with respect to telmisartan and rosuvastatin and a nonsignificant change in exposure to total ezetimibe and ezetimibe, which was not considered clinically significant without safety concerns. Furthermore, for the generalizability of the clinical effects, a large-scaled clinical study might be required in patients with hypertension and dyslipidemia.
Clinical Trial Registration
ClinicalTrials.gov registry number: NCT03802526.


Similar content being viewed by others
References
American Diabetes A. 10. Cardiovascular disease and risk management: standards of medical care in diabetes-2020. Diabetes Care. 2020;43(Suppl 1):S111–34. https://doi.org/10.2337/dc20-S010.
Thom S, Poulter N, Field J, Patel A, Prabhakaran D, Stanton A, et al. Effects of a fixed-dose combination strategy on adherence and risk factors in patients with or at high risk of CVD: the UMPIRE randomized clinical trial. JAMA. 2013;310(9):918–29. https://doi.org/10.1001/jama.2013.277064.
Bakris GL, Weir MR, Study of H, the Efficacy of Lotrel in Diabetes I. Achieving goal blood pressure in patients with type 2 diabetes: conventional versus fixed-dose combination approaches. J Clin Hypertens (Greenwich). 2003;5(3):202–9. https://doi.org/10.1111/j.1524-6175.2002.2041.x.
Yamada A, Maeda K, Ishiguro N, Tsuda Y, Igarashi T, Ebner T, et al. The impact of pharmacogenetics of metabolic enzymes and transporters on the pharmacokinetics of telmisartan in healthy volunteers. Pharmacogenet Genom. 2011;21(9):523–30. https://doi.org/10.1097/FPC.0b013e3283482502.
Kim CH, An H, Kim SH, Shin D. Pharmacokinetic and pharmacodynamic interaction between ezetimibe and rosuvastatin in healthy male subjects. Drug Des Dev Ther. 2017;11:3461–9. https://doi.org/10.2147/DDDT.S146863.
Konig J, Muller F, Fromm MF. Transporters and drug-drug interactions: important determinants of drug disposition and effects. Pharmacol Rev. 2013;65(3):944–66. https://doi.org/10.1124/pr.113.007518.
Wang X, Zhao X, Li L, Yao H, Jiang Y, Zhang J. Effects of combination of ezetimibe and rosuvastatin on coronary artery plaque in patients with coronary heart disease. Heart Lung Circ. 2016;25(5):459–65. https://doi.org/10.1016/j.hlc.2015.10.012.
Nickenig G. Should angiotensin II receptor blockers and statins be combined? Circulation. 2004;110(8):1013–20. https://doi.org/10.1161/01.CIR.0000139857.85424.45.
Son M, Kim Y, Lee D, Roh H, Son H, Guk J, et al. Pharmacokinetic interaction between rosuvastatin and telmisartan in healthy Korean male volunteers: a randomized, open-label, two-period, crossover, multiple-dose study. Clin Ther. 2014;36(8):1147–58. https://doi.org/10.1016/j.clinthera.2014.06.007.
Kiang TK, Ensom MH, Chang TK. UDP-glucuronosyltransferases and clinical drug–drug interactions. Pharmacol Ther. 2005;106(1):97–132. https://doi.org/10.1016/j.pharmthera.2004.10.013.
Son M, Guk J, Kim Y, Woo Chae D, Heo YA, Soh D, et al. Pharmacokinetic interaction between rosuvastatin, telmisartan, and amlodipine in healthy Male Korean subjects: a randomized, open-label, multiple-dose, 2-period crossover study. Clin Ther. 2016;38(8):1845–57. https://doi.org/10.1016/j.clinthera.2016.06.011.
Birmingham BK, Bujac SR, Elsby R, Azumaya CT, Zalikowski J, Chen Y, et al. Rosuvastatin pharmacokinetics and pharmacogenetics in Caucasian and Asian subjects residing in the United States. Eur J Clin Pharmacol. 2015;71(3):329–40. https://doi.org/10.1007/s00228-014-1800-0.
Wu HF, Hristeva N, Chang J, Liang X, Li R, Frassetto L, et al. Rosuvastatin pharmacokinetics in asian and white subjects wild type for both OATP1B1 and BCRP under control and inhibited conditions. J Pharm Sci. 2017;106(9):2751–7. https://doi.org/10.1016/j.xphs.2017.03.027.
Hu M, Lee HK, To KK, Fok BS, Wo SK, Ho CS, et al. Telmisartan increases systemic exposure to rosuvastatin after single and multiple doses, and in vitro studies show telmisartan inhibits ABCG2-mediated transport of rosuvastatin. Eur J Clin Pharmacol. 2016;72(12):1471–8. https://doi.org/10.1007/s00228-016-2130-1.
To KK, Tomlinson B. Targeting the ABCG2-overexpressing multidrug resistant (MDR) cancer cells by PPARgamma agonists. Br J Pharmacol. 2013;170(5):1137–51. https://doi.org/10.1111/bph.12367.
Stangier J, Su CA, van Heiningen PN, Meinicke T, van Lier JJ, de Bruin H, et al. Inhibitory effect of telmisartan on the blood pressure response to angiotensin II challenge. J Cardiovasc Pharmacol. 2001;38(5):672–85. https://doi.org/10.1097/00005344-200111000-00004.
Yang J, Li LJ, Wang K, He YC, Sheng YC, Xu L, et al. Race differences: modeling the pharmacodynamics of rosuvastatin in Western and Asian hypercholesterolemia patients. Acta Pharmacol Sin. 2011;32(1):116–25. https://doi.org/10.1038/aps.2010.169.
Acknowledgements
The investigation was conducted at the Clinical Trials Center, Gachon University Gil Medical Center.
Funding
This study was funded by Navipharm Co., Ltd. This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare, Republic of Korea (grant numbers: HI17C1919 and HI14C2750) and by Gachon University Gil Medical Center (Grant number: FRD2019-11). The journal’s publication fee was funded by the corresponding author (Dongseong Shin).
Authorship
All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Disclosures
Sol Ip Kang is an employee of Navipharm Co., Ltd. The other authors (Chang Hee Kim and Dongseong Shin) have no conflicts of interest to disclose.
Compliance with Ethics Guidelines
This clinical study was conducted in accordance with the recommendations of the Korean Good Clinical Practice and the 1964 Helsinki Declaration and its later amendments (ClinicalTrials.gov registry number: NCT03802526). The Institutional Review Board (IRB) of Gachon University Gil Medical Center approved this protocol and the informed consent form for this study (IRB approval number: GAIRB2019-055). Written informed consent forms were obtained from all participants before enrollment.
Data Availability
The datasets generated during the current study are available from the corresponding author on reasonable request.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kim, C.H., Kang, S.I. & Shin, D. Pharmacokinetic Interaction Between Telmisartan and Rosuvastatin/Ezetimibe After Multiple Oral Administration in Healthy Subjects. Adv Ther 38, 1094–1105 (2021). https://doi.org/10.1007/s12325-020-01592-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12325-020-01592-8