Abstract
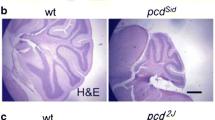
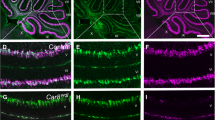
Degenerative effects of nerve tissues are often accompanied by changes in vascularization. In this regard, knowledge about hereditary cerebellar degeneration is limited. In this study, we compared the vascularity of the individual cerebellar components of 3-month-old wild-type mice (n = 8) and Purkinje cell degeneration (pcd) mutant mice, which represent a model of hereditary cerebellar degeneration (n = 8). Systematic random samples of tissue sections were processed, and laminin was immunostained to visualize microvessels. A computer-assisted stereology system was used to quantify microvessel parameters including total number, total length, and associated densities in cerebellar layers. Our results in pcd mice revealed a 45% (p < 0.01) reduction in the total volume of the cerebellum, a 28% (p < 0.05) reduction in the total number of vessels and a lower total length, approaching 50% (p < 0.001), compared to the control mice. In pcd mutants, cerebellar degeneration is accompanied by significant reduction in the microvascular network that is proportional to the cerebellar volume reduction therefore does not change density of in the cerebellar gray matter of pcd mice.


Similar content being viewed by others
Data Availability
The authors confirm that the data supporting the findings of this study are available within the article and its supplementary materials.
References
Qu W, Johnson A, Kim JH, Lukowicz A, Svedberg D, Cvetanovic M. Inhibition of colony-stimulating factor 1 receptor early in disease ameliorates motor deficits in SCA1 mice. J Neuroinflamm. 2017;14:107. https://doi.org/10.1186/s12974-017-0880-z.
Gyengesi E, Liang HZ, Millington C, Sonego S, Sirijovski D, Gunawardena D, et al. Investigation into the effects of tenilsetam on markers of neuroinflammation in GFAP-IL6 mice. Pharm Res. 2018;35:15. https://doi.org/10.1007/s11095-017-2326-9.
Wachter C, Eiden LE, Naumann N, Depboylu C, Weihe E. Loss of cerebellar neurons in the progression of lentiviral disease: effects of CNS-permeant antiretroviral therapy. J Neuroinflamm. 2016;13:13. https://doi.org/10.1186/s12974-016-0726-0.
Castrogiovanni P, Sanfilippo C, Imbesi R, Maugeri G, Lo Furno D, Tibullo D, et al. Brain CHID1 expression correlates with NRGN and CALB1 in healthy subjects and AD patients. Cells. 2021;10:18. https://doi.org/10.3390/cells10040882.
Patterson VL, Zullo AJ, Koenig C, Stoessel S, Jo H, Liu XR, et al. Neural-specific deletion of Htra2 causes cerebellar neurodegeneration and defective processing of mitochondrial OPA1. PLoS One. 2014;9:24. https://doi.org/10.1371/journal.pone.0115789.
Del Pilar C, Lebrón-Galán R, Pérez-Martín E, Pérez-Revuelta L, Ávila-Zarza CA, Alonso JR, et al. The selective loss of Purkinje cells induces specific peripheral immune alterations. Front Cell Neurosci. 2021;15:773696. https://doi.org/10.3389/fncel.2021.773696.
Kolinko Y, Krakorova K, Cendelin J, Tonar Z, Kralickova M. Microcirculation of the brain: morphological assessment in degenerative diseases and restoration processes. Rev Neurosci. 2015;26:75–93. https://doi.org/10.1515/revneuro-2014-0049.
Skaaraas G, Melbye C, Puchades MA, Leung DSY, Jacobsen O, Rao SB, et al. Cerebral amyloid angiopathy in a mouse model of Alzheimer’s disease associates with upregulated angiopoietin and downregulated hypoxia-inducible factor. J Alzheimers Dis. 2021;83:1651–63. https://doi.org/10.3233/jad-210571.
Fernandez-Klett F, Brandt L, Fernandez-Zapata C, Abuelnor B, Middeldorp J, Sluijs JA, et al. Denser brain capillary network with preserved pericytes in Alzheimer’s disease. Brain Pathol. 2020;30:1071–86. https://doi.org/10.1111/bpa.12897.
Roitbak T, Li L, Cunningham LA. Neural stem/progenitor cells promote endothelial cell morphogenesis and protect endothelial cells against ischemia via HIF-1 alpha-regulated VEGF signaling. J Cereb Blood Flow Metab. 2008;28:1530–42. https://doi.org/10.1038/jcbfm.2008.38.
Mitoma H, Manto M, Gandini J. Recent advances in the treatment of cerebellar disorders. Brain Sci. 2019;10. https://doi.org/10.3390/brainsci10010011.
Cendelin J. From mice to men: lessons from mutant ataxic mice. Cerebellum Ataxias. 2014;1:4. https://doi.org/10.1186/2053-8871-1-4.
Cendelin J, Cvetanovic M, Gandelman M, Hirai H, Orr HT, Pulst SM, et al. Consensus paper: strengths and weaknesses of animal models of spinocerebellar ataxias and their clinical implications. Cerebellum. 2022;21:452–81. https://doi.org/10.1007/s12311-021-01311-1.
Kolinko Y, Cendelin J, Kralickova M, Tonar Z. Smaller absolute quantities but greater relative densities of microvessels are associated with cerebellar degeneration in lurcher mice. Front Neuroanat. 2016;10:35. https://doi.org/10.3389/fnana.2016.00035.
Babuska V, Houdek Z, Tuma J, Purkartova Z, Tumova J, Kralickova M, et al. Transplantation of embryonic cerebellar grafts improves gait parameters in ataxic lurcher mice. Cerebellum. 2015;14:632–41. https://doi.org/10.1007/s12311-015-0656-x.
Cendelin J, Purkartova Z, Kubik J, Ulbricht E, Tichanek F, Kolinko Y. Long-term development of embryonic cerebellar grafts in two strains of lurcher mice. Cerebellum. 2018;17:428–37. https://doi.org/10.1007/s12311-018-0928-3.
Purkartova Z, Tichanek F, Kolinko Y, Cendelin J. Embryonic cerebellar graft morphology differs in two mouse models of cerebellar degeneration. Cerebellum. 2019;18:855–65. https://doi.org/10.1007/s12311-019-01067-9.
Fernandez-Gonzalez A, La Spada AR, Treadaway J, Higdon JC, Harris BS, Sidman RL, et al. Purkinje cell degeneration (pcd) phenotypes caused by mutations in the axotomy-induced gene, Nna1. Science. 2002;295:1904–6. https://doi.org/10.1126/science.1068912.
Kyuhou S, Kato N, Gemba H. Emergence of endoplasmic reticulum stress and activated microglia in Purkinje cell degeneration mice. Neurosci Lett. 2006;396:91–6. https://doi.org/10.1016/j.neulet.2005.11.023.
Chakrabarti L, Eng J, Ivanov N, Garden GA, La Spada AR. Autophagy activation and enhanced mitophagy characterize the Purkinje cells of pcd mice prior to neuronal death. Mol Brain. 2009;2:24. https://doi.org/10.1186/1756-6606-2-24.
Baltanas FC, Berciano MT, Valero J, Gomez C, Diaz D, Alonso JR, et al. Differential glial activation during the degeneration of Purkinje cells and mitral cells in the PCD mutant mice. Glia. 2013;61:254–72. https://doi.org/10.1002/glia.22431.
Mullen RJ, Eicher EM, Sidman RL. Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci U S A. 1976;73:208–12.
Ghetti B, Norton J, Triarhou LC. Nerve cell atrophy and loss in the inferior olivary complex of “Purkinje cell degeneration” mutant mice. J Comp Neurol. 1987;260:409–22. https://doi.org/10.1002/cne.902600307.
Triarhou LC. Biological clues on neuronal degeneration based on theoretical fits of decay patterns: towards a mathematical neuropathology. Folia Neuropathol. 2010;48:3–10.
Triarhou LC, Norton J, Ghetti B. Anterograde transsynaptic degeneration in the deep cerebellar nuclei of Purkinje cell degeneration (pcd) mutant mice. Exp Brain Res. 1987;66:577–88.
Blanks JC, Mullen RJ, LaVail MM. Retinal degeneration in the pcd cerebellar mutant mouse. II. Electron microscopic analysis. J Comp Neurol. 1982;212:231–46. https://doi.org/10.1002/cne.902120303.
LaVail MM, Blanks JC, Mullen RJ. Retinal degeneration in the pcd cerebellar mutant mouse .I. Light microscopic and autoradiographic analysis. J Comp Neurol. 1982;212:217–30. https://doi.org/10.1002/cne.902120302.
Blanks JC, Spee C. Retinal degeneration in the pcd/pcd mutant mouse: accumulation of spherules in the interphotoreceptor space. Exp Eye Res. 1992;54:637–44.
O’Gorman S, Sidman RL. Degeneration of thalamic neurons in “Purkinje cell degeneration” mutant mice. I. Distribution of neuron loss. J Comp Neurol. 1985;234:277–97. https://doi.org/10.1002/cne.902340302.
Kolinko Y, Marsalova L, Pena SP, Kralickova M, Mouton PR. Stereological changes in microvascular parameters in hippocampus of a transgenic rat model of Alzheimer’s disease. J Alzheimers Dis. 2021;84:249–60. https://doi.org/10.3233/jad-210738.
Kleiter N, Lametschwandtner A. Microvascularization of the cerebellum in the turtle, Pseudemys scripta elegans (Reptilia). A scanning electron microscope study of microvascular corrosion casts, including stereological measurements. Anat Embryol (Berl). 1995;191:145–53.
Mouton PR. Principles and practices of unbiased stereology: an introduction for bioscientists. Baltimore, Maryland, USA: Johns Hopkins University Press; 2002.
Mouton PR. Unbiased stereology: a concise guide. Baltimore, Maryland, USA: Johns Hopkins University Press; 2011.
Sterio DC. The unbiased estimation of number and sizes of arbitrary particles using the disector. J Microsc. 1984;134:127–36. https://doi.org/10.1111/j.1365-2818.1984.tb02501.x.
Nyengaard JR, Marcussen N. The number of glomerular capillaries estimated by an unbiased and efficient stereological method. J Microsc. 1993;171:27–37. https://doi.org/10.1111/j.1365-2818.1993.tb03356.x.
Lee GD, Aruna JH, Barrett PM, Lei DL, Ingram DK, Mouton PR. Stereological analysis of microvascular parameters in a double transgenic model of Alzheimer’s disease. Brain Res Bull. 2005;65:317–22. https://doi.org/10.1016/j.brainresbull.2004.11.024.
West MJ. Space balls revisited: stereological estimates of length with virtual isotropic surface probes. Front Neuroanat. 2018;12:49. https://doi.org/10.3389/fnana.2018.00049.
Isaacs KR, Anderson BJ, Alcantara AA, Black JE, Greenough WT. Exercise and the brain: angiogenesis in the adult rat cerebellum after vigorous physical activity and motor skill learning. J Cereb Blood Flow Metab. 1992;12:110–9. https://doi.org/10.1038/jcbfm.1992.14.
Gundersen HJ, Jensen EB, Kieu K, Nielsen J. The efficiency of systematic sampling in stereology–reconsidered. J Microsc. 1999;193:199–211.
Wang TY, Morgan JI. The Purkinje cell degeneration (pcd) mouse: an unexpected molecular link between neuronal degeneration and regeneration. Brain Res. 2007;1140:26–40. https://doi.org/10.1016/j.brainres.2006.07.065.
Rhyu IJ, Bytheway JA, Kohler SJ, Lange H, Lee KJ, Boklewski J, et al. Effects of aerobic exercise training on cognitive function and cortical vascularity in monkeys. Neuroscience. 2010;167:1239–48. https://doi.org/10.1016/j.neuroscience.2010.03.003.
Swain RA, Harris AB, Wiener EC, Dutka MV, Morris HD, Theien BE, et al. Prolonged exercise induces angiogenesis and increases cerebral blood volume in primary motor cortex of the rat. Neuroscience. 2003;117:1037–46. https://doi.org/10.1016/s0306-4522(02)00664-4.
Caddy KW, Biscoe TJ. Structural and quantitative studies on the normal C3H and Lurcher mutant mouse. Philos Trans R Soc Lond B Biol Sci. 1979;287:167–201.
Vernet-der Garabedian B, Lemaigre-Dubreuil Y, Delhaye-Bouchaud N, Mariani J. Abnormal IL-1beta cytokine expression in the cerebellum of the ataxic mutant mice staggerer and lurcher. Brain Res Mol Brain Res. 1998;62:224–7.
Funding
This work was supported by the Cooperatio Program, research area MED/DIAG.
Author information
Authors and Affiliations
Contributions
Dr. Kolinko performed the histological morphometry, the data analysis, and the literature search and wrote the manuscript. Dr. Cendelin was responsible for the mouse brain collections and provided support during the literature search and writing of the manuscript. All the authors contributed equally to the generation of ideas, general editing, and revising the text critically for important intellectual contention. The final manuscript preparation was conducted by the first author.
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kolinko, Y., Kralickova, M. & Cendelin, J. Reduction of Microvessel Number and Length in the Cerebellum of Purkinje Cell Degeneration Mice. Cerebellum 23, 471–478 (2024). https://doi.org/10.1007/s12311-023-01556-y
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-023-01556-y