Summary
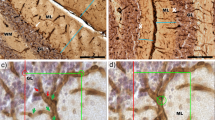
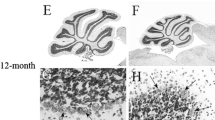
The genetically-determined loss of Purkinje cells (PCs) in ‘Purkinje cell degeneration’ (pcd) mutant mice results in the loss of presynaptic afferents to the deep cerebellar nuclei (DCN). This deafferentation takes place between postnatal day (P)17 and P45, i.e. after the maturation of cerebellar circuitry. We examined the DCN of normal and pcd mutant mice by quantitative light microscopic methods to determine whether neuronal atrophy or loss in the DCN take place during and after the loss of their input from the PCs. Neuronal diameters in control mice were 16.4±0.72 μm (mean±S.D.) at P23 and 15.6±0.64 μm at P300. The respective values in pcd mutant mice were 15.7±0.58 μm and 13.5±0.24 μm. Diameters in 300-day-old mutants were significantly smaller than those in both age-matched controls and 23-day-old mutants (P< 0.001). Neuronal populations in the DCN of control mice were 10,167± 949 at P23 and 10,429±728 at P300. The respective values in mutants were 9,436±1,366 and 7,424±1,324. There was a significant difference of 29% [95% confidence limits: 9–45%] between 300-day-old mutants and age-matched controls (P<0.01), and a significant loss of 21% [95% confidence limits: 4–36%] in 300-day-old mutants with respect to 23-day-old mutants (P<0.05). The total volume of the DCN was 22% less in 300-day-old mutants in relation to 23-day-old mutants (P< 0.05). These findings support the idea that the stability of DCN neurons in the mature cerebellum depends in part on the synaptic input from PCs.
Similar content being viewed by others
References
Abercrombie M (1946) Estimation of nuclear population from microtome sections. Anat Rec 94: 239–247
Asanuma C, Thach WT, Jones EG (1983) Distribution of cerebellar terminations and their relation to other afferent terminations in the ventral lateral thalamic region of the monkey. Brain Res Rev 5: 237–265
Brodal A (1982) Anterograde and retrograde degeneration of nerve cells in the central nervous system. In: Haymaker W, Adams RD (eds) Histology and histopathology of the nervous system. Charles C Thomas Publisher, Springfield, pp 276–362
Caddy KWT, Biscoe TJ (1979) Structural and quantitative studies on the normal C3H and lurcher mutant mouse. Philos Trans R Soc Lond (Biol) 287: 167–201
Chan-Palay V (1977) Cerebellar dentate nucleus: organization, cytology and transmitters. Springer, Berlin Heidelberg New York
Clark WE Le Gros, Penman GG (1934) Projection of retina in lateral geniculate body. Proc R Soc Lond (Biol) 114: 291–313
Colquhoun D (1971) Lectures on biostatistics. Oxford University Press, Oxford, pp 293–297
Cowan WM (1970) Anterograde and retrograde transneuronal degeneration in the central and peripheral nervous system. In: Nauta WJH, Ebbesson SOE (eds) Contemporary research methods in neuroanatomy. Springer, Berlin Heidelberg New York, pp 217–251
Cullen MJ, Kaiserman-Abramof IR (1976) Cytological organization of the dorsal lateral geniculate nuclei in mutant anophthalmic and postnatally enucleated mice. J Neurocytol 5: 407–424
DeLong GR, Sidman RL (1962) Effects of eye removal at birth on histogenesis of the mouse superior colliculus: an autoradiographic analysis with tritiated thymidine. J Comp Neurol 118: 205–233
Fonnum F, Walberg F (1973) An estimation of the concentration of gamma-aminobutyric acid and glutamate decarboxylase in the inhibitory Purkinje axon terminals in the cat. Brain Res 54: 115–127
Ghetti B, Horoupian DS, Wiśniewski HM (1972) Transsynaptic response of the lateral geniculate nucleus and the pattern of degeneration of the nerve terminals in the rhesus monkey after eye enucleation. Brain Res 45: 31–48
Ghetti B, Horoupian DS, Wiśniewski HM (1975) Acute and long-term transneuronal response of dendrites of lateral geniculate neurons following transection of the primary visual afferent pathway. Adv Neurol 12: 401–424
Ghetti B, Norton J, Triarhou LC (1986) Anterograde transneuronal atrophy and loss in the deep cerebellar nuclei of ‘Purkinje cell degeneration’ mutant mice. Abstr Xth Int Congr Neuropathol. Gotab, Stockholm, p 385
Ghetti B, Norton J, Triarhou LC (1987) Nerve cell atrophy and loss in the inferior olivary complex of Purkinje cell degeneration (pcd) mutant mice. J Comp Neurol (in press)
Greer CA, Shepherd GM (1982) Mitral cell degeneration and sensory function in the neurological mutant mouse Purkinje cell degeneration. Brain Res 235: 156–161
Gudden BA von (1874) Über die Kreuzung der Fasern im Chiasma nervorum opticorum. Albrecht von Graefes Arch Ophthalmol Verein Z Arch Augenheilkd 20: 249–267
Herrup K, Mullen RJ (1979) Regional variation and absence of large neurons in the cerebellum of the staggerer mouse. Brain Res 172: 1–12
Heumann D, Rabinowicz Th (1980) Postnatal development of the dorsal lateral geniculate nucleus in the normal and enucleated albino mouse. Exp Brain Res 38: 75–85
Karnovsky MJ (1965) A formaldehyde-glutaraldehyde fixative of high osmolarity for use in electron microscopy. J Cell Biol 27: 137A-138A
Konigsmark BW (1970) Methods for the counting of neurons. In: Nauta WJH, Ebbesson SOE (eds) Contemporary research methods in neuroanatomy. Springer, Berlin Heidelberg New York, pp 315–340
LaVail MW, Blanks JC, Mullen RJ (1982) Retinal degeneration in the pcd cerebellar mutant mouse. I. Light microscopic and autoradiographic analysis. J Comp Neurol 212: 217–230
Mehler WR, Nauta WJH (1974) Connections of the basal ganglia and of the cerebellum. Confin Neurol (Basel) 36: 205–222
Monakow C von (1882) Über einige durch Extirpation circumscripter Hirnrindenregionen bedingte Entwicklungshemmungen des Kaninchengehirns. Arch Psychiatr Nervenkr Verein Z Gesamte Neurol Psychiatr 12: 141–156
Mullen RJ, Eicher EM, Sidman RL (1976) Purkinje cell degeneration, a new neurological mutation in the mouse. Proc Natl Acad Sci USA 73: 208–212
O'Gorman S, Sidman RL (1985) Degeneration of thalamic neurons in ‘Purkinje cell degeneration’ mutant mice. I. Distribution of neuron loss. J Comp Neurol 234: 277–297
Palkovits M, Mezey É, Hámori J, Szentágothai J (1977) Quantitative histological analysis of the cerebellar nuclei in the cat. I. Numerical data on cells and on synapses. Exp Brain Res 28: 189–209
Peduzzi JD, Crossland WJ (1983) Anterograde transneuronal degeneration in the ectomamillary nucleus and ventral lateral geniculate nucleus of the chick. J Comp Neurol 213: 287–300
Rinvik E, Grofová I (1974) Cerebellar projections to the nuclei ventralis lateralis and ventralis anterior thalami. Experimental electron microscopical and light microscopical studies in the cat. Anat Embryol (Berl) 146: 95–111
Roffler-Tarlov S, Herrup K (1981) Quantitative examination of the deep cerebellar nuclei in the staggerer mutant mouse. Brain Res 215: 49–59
Ryan BF, Joiner BL, Ryan TA Jr (1985) Minitab handbook, 2nd edn. Duxbury Press, Boston, pp 347–350
Schoene WC (1985) Degenerative diseases of the central nervous system. In: Davis RL, Robertson DM (eds) Textbook of neuropathology. Williams and Wilkins, Baltimore, pp 788–823
Sidman RL, Lane PW, Dickie MM (1962) Staggerer, a new mutation in the mouse affecting the cerebellum. Science 137: 610–612
Smeulders AWM, Dorst L (1985) Measurement issues in morphometry. Anal Quant Cytol Histol 7: 242–249
Sokal RR, Rohlf FJ (1981) Biometry. WH Freeman and Company, New York
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Triarhou, L.C., Norton, J. & Ghetti, B. Anterograde transsynaptic degeneration in the deep cerebellar nuclei of Purkinje cell degeneration (pcd) mutant mice. Exp Brain Res 66, 577–588 (1987). https://doi.org/10.1007/BF00270691
Received:
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00270691