Abstract
Purpose
To test the short- and long-term effects of Tenilsetam on chronic neuroinflammation in the GFAP-IL6 mouse.
Methods
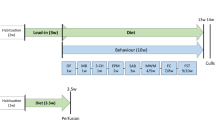
From 3 months of age, GFAP-IL6 mice were divided into 2 groups and fed with Tenilsetam enriched food pellets or control food pellets, respectively, for either 5 or 15 months. Total numbers of Iba-1+ microglia, TSPO+ cells were determined using an unbiased stereological method. Levels of methylglyoxal and TNF-α in the cerebellar homogenate were tested using HPLC and ELISA, respectively.
Results
Tenilsetam decreased the total number of Iba-1+ microglia in both the cerebellum and the hippocampus of GFAP-IL6 mice at 8 months and in the cerebellum at 18 months. In the cerebellum, it decreased the density of microglia in GFAP-IL6 mice to a similar level after 5 and 15 months’ feeding. Tenilsetam prevented the volume loss of the cerebellum at 8 months. It also significantly decreased TNF-α in the cerebellum of GFAP-IL6 mice to a similar level of WT mice after 15 months of feeding.
Conclusion
Tenilsetam has anti-inflammatory effects evidenced by the decreased number of microglia in both the cerebellum and hippocampus, and decreased TNF-α levels in the GFAP-IL6 Tenilsetam fed animals.









Similar content being viewed by others
Abbreviations
- ABC:
-
Avidin/biotin complex
- AD:
-
Alzheimer’s disease
- AGE:
-
Advanced glycation endproduct
- ANOVA:
-
Analysis of variance
- AP1:
-
Activator protein 1
- ATP:
-
Adenosine triphosphate
- CLU:
-
Clusterin
- CNS:
-
Central nervous system
- COXs:
-
Cyclooxygenases
- CR1:
-
Complement receptor 1
- DAB:
-
3,3′-diaminobenzidine
- DTH:
-
Delayed-type hypersensitivity
- FJT:
-
Favourability judgement task
- GFAP:
-
Glial fibrillary acidic protein
- GFAP-L6:
-
Glial fibrillary acidic protein-interleukin 6
- H2O2 :
-
Hydrogen peroxide
- Iba-1:
-
Ionized calcium-binding adapter molecule 1
- IL1:
-
Interleukin 1
- IL1β:
-
Interleukin 1β
- IL6:
-
Interleukin 6
- LPS:
-
Lipopolysaccharide
- MGO:
-
Methylglyoxal
- NF-κB:
-
Nuclear factor kappa-light-chain-enhancer of activated B cells
- NSAIDs:
-
Non-steroidal anti-inflammatory drugs
- PBS:
-
Phosphate-buffered saline
- RAGE:
-
Receptor of advanced glycation endproduct
- SD:
-
Standard deviation
- TNF- α:
-
Tumor necrosis factor α
- TREM2:
-
Triggering receptor expressed on myeloid cells 2
References
Mrak RE, Griffin WS. Glia and their cytokines in progression of neurodegeneration. Neurobiol Aging. 2005;26(3):349–54.
Retz W, Gsell W, Münch G, Rosler M, Riederer P. Free radicals in Alzheimer's disease. J Neural Transm Suppl. 1998;54:221–36.
Ahmed N, Ahmed U, Thornalley PJ, Hager K, Fleischer G, Münch G. Protein glycation, oxidation and nitration adduct residues and free adducts of cerebrospinal fluid in Alzheimer's disease and link to cognitive impairment. J Neurochem. 2005;92(2):255–63.
Münch G, Thome J, Foley P, Schinzel R, Riederer P. Advanced glycation endproducts in ageing and Alzheimer's disease. Brain Res Brain Res Rev. 1997;23(1–2):134–43.
Fuller S, Münch G, Steele M. Activated astrocytes: a therapeutic target in Alzheimer's disease? Expert Rev Neurother. 2009;9(11):1585–94.
Fuller S, Steele M, Münch G. Activated astroglia during chronic inflammation in Alzheimer's disease--do they neglect their neurosupportive roles? Mutat Res. 2010;690(1–2):40–9.
Patel A, Rees SD, Kelly MA, Bain SC, Barnett AH, Prasher A, et al. Genetic variants conferring susceptibility to Alzheimer's disease in the general population; do they also predispose to dementia in Down's syndrome. BMC Res Notes. 2014;7(1):42.
Shaftel SS, Carlson TJ, Olschowka JA, Kyrkanides S, Matousek SB, O'Banion MK. Chronic interleukin-1beta expression in mouse brain leads to leukocyte infiltration and neutrophil-independent blood brain barrier permeability without overt neurodegeneration. J Neurosci Off J Soc Neurosci. 2007;27(35):9301–9.
Campbell IL, Abraham CR, Masliah E, Kemper P, Inglis JD, Oldstone MB, et al. Neurologic disease induced in transgenic mice by cerebral overexpression of interleukin 6. Proc Natl Acad Sci U S A. 1993;90(21):10061–5.
Carrasco J, Giralt M, Penkowa M, Stalder AK, Campbell IL, Hidalgo J. Metallothioneins are upregulated in symptomatic mice with astrocyte-targeted expression of tumor necrosis factor-alpha. Exp Neurol. 2000;163(1):46–54.
Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proc Natl Acad Sci U S A. 1997;94(4):1500–5.
Wang XM, Wu TX, Hamza M, Ramsay ES, Wahl SM, Dionne RA. Rofecoxib modulates multiple gene expression pathways in a clinical model of acute inflammatory pain. Pain. 2007;128(1–2):136–47.
Bour AM, Westendorp RG, Laterveer JC, Bollen EL, Remarque EJ. Interaction of indomethacin with cytokine production in whole blood. Potential mechanism for a brain-protective effect. Exp Gerontol. 2000;35(8):1017–24.
Strassburger M, Braun H, Reymann KG. Anti-inflammatory treatment with the p38 mitogen-activated protein kinase inhibitor SB239063 is neuroprotective, decreases the number of activated microglia and facilitates neurogenesis in oxygen-glucose-deprived hippocampal slice cultures. Eur J Pharmacol. 2008;592(1–3):55–61.
Sun YX, Dai DK, Liu R, Wang T, Luo CL, Bao HJ, et al. Therapeutic effect of SN50, an inhibitor of nuclear factor-kappaB, in treatment of TBI in mice. Neurol Sci. 2013;34(3):345–55.
Maczurek A, Hager K, Kenklies M, Sharman M, Martins R, Engel J, et al. Lipoic acid as an anti-inflammatory and neuroprotective treatment for Alzheimer's disease. Adv Drug Deliv Rev. 2008;60(13–14):1463–70.
Steele ML, Fuller S, Patel M, Kersaitis C, Ooi L, Münch G. Effect of Nrf2 activators on release of glutathione, cysteinylglycine and homocysteine by human U373 astroglial cells. Redox Biol. 2013;1:441–5.
Apetz N, Münch G, Govindaraghavan S, Gyengesi E. Natural compounds and plant extracts as therapeutics against chronic inflammation in Alzheimer's disease--a translational perspective. CNS Neurol Disord Drug Targets. 2014;13(7):1175–91.
Venigalla M, Gyengesi E, Münch G. Curcumin and Apigenin - novel and promising therapeutics against chronic neuroinflammation in Alzheimer's disease. Neural Regen Res. 2015;10(8):1181–5.
Ullah F, Liang A, Rangel A, Gyengesi E, Niedermayer G, Munch G. High bioavailability curcumin: an anti-inflammatory and neurosupportive bioactive nutrient for neurodegenerative diseases characterized by chronic neuroinflammation. Arch Toxicol. 2017;91(4):1623–34.
Steiner N, Balez R, Karunaweera N, Lind JM, Munch G, Ooi L. Neuroprotection of Neuro2a cells and the cytokine suppressive and anti-inflammatory mode of action of resveratrol in activated RAW264.7 macrophages and C8-B4 microglia. Neurochem Int. 2016;95:46–54.
Venigalla M, Sonego S, Gyengesi E, Sharman MJ, Münch G. Novel promising therapeutics against chronic neuroinflammation and neurodegeneration in Alzheimer's disease. Neurochem Int. 2016;95:63–74.
Raju R, Gunawardena D, Ahktar MA, Low M, Reddell P, Munch G. Anti-Inflammatory Chemical Profiling of the Australian Rainforest Tree Alphitonia petriei (Rhamnaceae). Molecules. 2016;21(11). https://doi.org/10.3390/molecules21111521.
Akhtar MA, Raju R, Beattie KD, Bodkin F, Munch G. Medicinal Plants of the Australian Aboriginal Dharawal People Exhibiting Anti-Inflammatory Activity. Evid Based Complement Alternat Med. 2016;2016:2935403.
Ihl R, Perisic I, Maurer K, Dierks T. Effects of 3 months treatment with tenilsetam in patients suffering from dementia of Alzheimer type (DAT). J Neural Transm. 1989;1:84–5.
Saletu B, Semlitsch HV, Anderer P, Resch F, Presslich O, Schuster P. Psychophysiological research in psychiatry and neuropsychopharmacology. II. The investigation of antihypoxidotic/nootropic drugs (tenilsetam and co-dergocrine-mesylate) in elderlies with the Viennese Psychophysiological Test-System (VPTS). Methods Find Exp Clin Pharmacol. 1989;11(1):43–55.
Webster J, Urban C, Berbaum K, Loske C, Alpar A, Gartner U, et al. The carbonyl scavengers aminoguanidine and tenilsetam protect against the neurotoxic effects of methylglyoxal. Neurotox Res. 2005;7(1–2):95–101.
Münch G, Mayer S, Michaelis J, Hipkiss AR, Riederer P, Muller R, et al. Influence of advanced glycation end-products and AGE-inhibitors on nucleation-dependent polymerization of beta-amyloid peptide. Biochim Biophys Acta. 1997;1360(1):17–29.
Münch G, Taneli Y, Schraven E, Schindler U, Schinzel R, Palm D, et al. The cognition-enhancing drug tenilsetam is an inhibitor of protein crosslinking by advanced glycosylation. J Neural Transm Park Dis Dement Sect. 1994;8(3):193–208.
Münch G, Schicktanz D, Behme A, Gerlach M, Riederer P, Palm D, et al. Amino acid specificity of glycation and protein-AGE crosslinking reactivities determined with a dipeptide SPOT library. Nat Biotechnol. 1999;17(10):1006–10.
Yan SD, Schmidt AM, Stern D. Alzheimer's disease: inside, outside, upside down. Biochem Soc Symp. 2001;67:15–22.
Bultmann A, Li Z, Wagner S, Gawaz M, Ungerer M, Langer H, et al. Loss of protease activity of ADAM15 abolishes protective effects on plaque progression in atherosclerosis. Int J Cardiol. 2011;152(3):382–5.
Chiang CS, Stalder A, Samimi A, Campbell IL. Reactive gliosis as a consequence of interleukin-6 expression in the brain: studies in transgenic mice. Dev Neurosci. 1994;16(3–4):212–21.
Millington C, Sonego S, Karunaweera N, Rangel A, Aldrich-Wright JR, Campbell IL, et al. Chronic neuroinflammation in Alzheimer's disease: new perspectives on animal models and promising candidate drugs. Biomed Res Int. 2014;2014:309129.
Bastide MF, Dovero S, Charron G, Porras G, Gross CE, Fernagut PO, et al. Immediate-early gene expression in structures outside the basal ganglia is associated to l-DOPA-induced dyskinesia. Neurobiol Dis. 2014;62:179–92.
West MJ. Getting started in stereology. Cold Spring Harb Protoc. 2013;2013(4):287–97.
McCloy RA, Rogers S, Caldon CE, Lorca T, Castro A, Burgess A. Partial inhibition of Cdk1 in G 2 phase overrides the SAC and decouples mitotic events. Cell Cycle. 2014;13(9):1400–12.
Gunawardena D, Shanmugam K, Low M, Bennett L, Govindaraghavan S, Head R, Ooi L, Münch G. Determination of anti-inflammatory activities of standardised preparations of plant- and mushroom-based foods. Eur J Nutr. 2014;53(1):335–43.
Dhananjayan K, Gunawardena D, Hearn N, Sonntag T, Moran C, Gyengesi E, et al. Activation of Macrophages and Microglia by Interferon-gamma and Lipopolysaccharide Increases Methylglyoxal Production: A New Mechanism in the Development of Vascular Complications and Cognitive Decline in Type 2 Diabetes Mellitus? J Alzheimers Dis. 2017;59(2):467–79.
Espinosa-Mansilla A, Duran-Meras I, Salinas F. High-performance liquid chromatographic-fluorometric determination of glyoxal, methylglyoxal, and diacetyl in urine by prederivatization to pteridinic rings. Anal Biochem. 1998;255(2):263–73.
Cosenza-Nashat M, Zhao ML, Suh HS, Morgan J, Natividad R, Morgello S, et al. Expression of the translocator protein of 18 kDa by microglia, macrophages and astrocytes based on immunohistochemical localization in abnormal human brain. Neuropathol Appl Neurobiol. 2009;35(3):306–28.
Srikanth V, Westcott B, Forbes J, Phan TG, Beare R, Venn A, et al. Methylglyoxal, cognitive function and cerebral atrophy in older people. J Gerontol A Biol Sci Med Sci. 2013;68(1):68–73.
Kuhla B, Luth H-J, Haferburg D, Boeck K, Arendt T, MÜNch G. Methylglyoxal, Glyoxal, and Their Detoxification in Alzheimer's Disease. Ann N Y Acad Sci. 2005;1043(1):211–6.
de Arriba SG, Krugel U, Regenthal R, Vissiennon Z, Verdaguer E, Lewerenz A, et al. Carbonyl stress and NMDA receptor activation contribute to methylglyoxal neurotoxicity. Free Radic Biol Med. 2006;40(5):779–90.
Münch G, Westcott B, Menini T, Gugliucci A. Advanced glycation endproducts and their pathogenic roles in neurological disorders. Amino Acids. 2012;42(4):1221–36.
Kuhla B, Loske C. Garcia De Arriba S, Schinzel R, Huber J, Münch G. Differential effects of "Advanced glycation endproducts" and beta-amyloid peptide on glucose utilization and ATP levels in the neuronal cell line SH-SY5Y. J Neural Transm. 2004;111(3):427–39.
Srikanth V, Maczurek A, Phan T, Steele M, Westcott B, Juskiw D, et al. Advanced glycation endproducts and their receptor RAGE in Alzheimer's disease. Neurobiol Aging. 2011;32(5):763–77.
Maczurek A, Shanmugam K, Münch G. Inflammation and the redox-sensitive AGE-RAGE pathway as a therapeutic target in Alzheimer's disease. Ann N Y Acad Sci. 2008;1126:147–51.
Liu GJ, Middleton RJ, Hatty CR, Kam WW, Chan R, Pham T, et al. The 18 kDa translocator protein, microglia and neuroinflammation. Brain Pathol. 2014;24(6):631–53.
Chen MK, Guilarte TR. Translocator protein 18 kDa (TSPO): molecular sensor of brain injury and repair. Pharmacol Ther. 2008;118(1):1–17.
Papadopoulos V, Lecanu L, Brown RC, Han Z, Yao ZX. Peripheral-type benzodiazepine receptor in neurosteroid biosynthesis, neuropathology and neurological disorders. Neuroscience. 2006;138(3):749–56.
Ravikumar B, Crawford D, Dellovade T, Savinainen A, Graham D, Liere P, et al. Differential efficacy of the TSPO ligands etifoxine and XBD-173 in two rodent models of Multiple Sclerosis. Neuropharmacology. 2016;108:229–37.
Rabbani N, Thornalley PJ. Measurement of methylglyoxal by stable isotopic dilution analysis LC-MS/MS with corroborative prediction in physiological samples. Nat Protoc. 2014;9(8):1969–79.
Thornalley PJ. Cell activation by glycated proteins. AGE receptors, receptor recognition factors and functional classification of AGEs. Cell Mol Biol (Noisy-le-grand). 1998;44(7):1013–23.
Xue J, Ray R, Singer D, Bohme D, Burz DS, Rai V, et al. The receptor for advanced glycation end products (RAGE) specifically recognizes methylglyoxal-derived AGEs. Biochemistry. 2014;53(20):3327–35.
Kang R, Loux T, Tang D, Schapiro NE, Vernon P, Livesey KM, et al. The expression of the receptor for advanced glycation endproducts (RAGE) is permissive for early pancreatic neoplasia. Proc Natl Acad Sci U S A. 2012;109(18):7031–6.
Xie J, Mendez JD, Mendez-Valenzuela V, Aguilar-Hernandez MM. Cellular signalling of the receptor for advanced glycation end products (RAGE). Cell Signal. 2013;25(11):2185–97.
Yin L, Dai Q, Jiang P, Zhu L, Dai H, Yao Z, Liu H, Ma X, Qu L, Jiang J. Manganese exposure facilitates microglial JAK2-STAT3 signaling and consequent secretion of TNF-a and IL-1beta to promote neuronal death. Neurotoxicology. 2017. https://doi.org/10.1016/j.neuro.2017.04.001.
Priller J, Flugel A, Wehner T, Boentert M, Haas CA, Prinz M, et al. Targeting gene-modified hematopoietic cells to the central nervous system: use of green fluorescent protein uncovers microglial engraftment. Nat Med. 2001;7(12):1356–61.
Bierhaus A, Stern DM, Nawroth PP. RAGE in inflammation: a new therapeutic target? Curr Opin Investig Drugs. 2006;7(11):985–91.
ACKNOWLEDGMENTS AND DISCLOSURES
The present study is supported by the Research Training Fund of Western Sydney University (to MV).
Author information
Authors and Affiliations
Corresponding author
Additional information
Guest Editor: Davide Brambilla
Erika Gyengesi and Huazheng Liang are equal first authors.
Rights and permissions
About this article
Cite this article
Gyengesi, E., Liang, H., Millington, C. et al. Investigation Into the Effects of Tenilsetam on Markers of Neuroinflammation in GFAP-IL6 Mice. Pharm Res 35, 22 (2018). https://doi.org/10.1007/s11095-017-2326-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s11095-017-2326-9