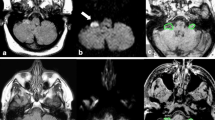
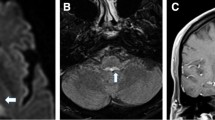
Abstract
We report atypical opsoclonus in a patient with multiple system atrophy and propose a mechanism based on the patterns of modulation by visual, vestibular, and saccadic and vergence stimulation. Firstly, the 6-Hz opsoclonus mostly in the vertical plane occurred only after the development of downbeat nystagmus in darkness without visual fixation. Even after a substantial build-up, visual suppression of the opsoclonus was immediate and complete. Furthermore, the latency for re-emergence of opsoclonus in darkness was greater when the duration of preceding visual fixation was longer. Secondly, the effect of preceding downbeat nystagmus on the development of opsoclonus was evaluated by changing the head position. The opsoclonus did not occur in the supine position when the downbeat nystagmus was absent. After horizontal head shaking, the opsoclonus in the vertical plane gradually evolved into horizontal plane and resumed its vertical direction again after vertical head shaking. Thirdly, any opsoclonus was not triggered by imaginary saccades in the supine position. Lastly, combined vergence and saccadic eye movements during the Müller paradigm did not induce opsoclonus. From these findings of modulation, we suggest that the opsoclonus observed in our patient was invoked by vestibular signals. When the function of the omnipause neurons and saccadic system was impaired, the centrally mediated vestibular eye velocity signals may activate the saccadic system to generate opsoclonus. These atypical patterns of opsoclonus, distinct from the classic opsoclonus frequently observed in para-neoplastic or para-infectious disorders, may be an unrevealing sign of degenerative brainstem or cerebellar disorders.




Similar content being viewed by others
References
Leigh RJ and Zee DS. The neurology of eye movements. OUP USA; 2015.
Ramat S, Leigh RJ, Zee DS, Optican LM. What clinical disorders tell us about the neural control of saccadic eye movements. Brain. 2007;130:10–35. https://doi.org/10.1093/brain/awl309.
Armangue T, Sabater L, Torres-Vega E, Martinez-Hernandez E, Arino H, Petit-Pedrol M, et al. Clinical and immunological features of opsoclonus-myoclonus syndrome in the era of neuronal cell surface antibodies. JAMA Neurol. 2016;73:417–24. https://doi.org/10.1001/jamaneurol.2015.4607.
Oh SY, Kim JS, Dieterich M. Update on opsoclonus-myoclonus syndrome in adults. J Neurol. 2019;266:1541–8. https://doi.org/10.1007/s00415-018-9138-7.
Ramat S, Leigh RJ, Zee DS, Optican LM. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res. 2005;160:89–106. https://doi.org/10.1007/s00221-004-1989-8.
Ramat S, Leigh RJ, Zee DS, Shaikh AG, Optican LM. Applying saccade models to account for oscillations. Prog Brain Res. 2008;171:123–30. https://doi.org/10.1016/S0079-6123(08)00616-X.
Shaikh AG, Ramat S, Optican LM, Miura K, Leigh RJ, Zee DS. Saccadic burst cell membrane dysfunction is responsible for saccadic oscillations. J Neuroophthalmol. 2008;28:329–36. https://doi.org/10.1097/WNO.0b013e31818eb3a5.
Shaikh AG, Zee DS, Optican LM, Miura K, Ramat S, Leigh RJ. The effects of ion channel blockers validate the conductance-based model of saccadic oscillations. Ann N Y Acad Sci. 2011;1233:58–63. https://doi.org/10.1111/j.1749-6632.2011.06130.x.
Zee DS, Robinson DA. A hypothetical explanation of saccadic oscillations. Ann Neurol. 1979;5:405–14. https://doi.org/10.1002/ana.410050502.
Wong AM, Musallam S, Tomlinson RD, Shannon P, Sharpe JA. Opsoclonus in three dimensions: oculographic, neuropathologic and modelling correlates. J Neurol Sci. 2001;189:71–81.
Scholz J, Vieregge P, Ruff C. Paraneoplastic opsoclonus-myoclonus syndrome in metastatic ovarian carcinoma. J Neurol Neurosurg Psychiatry. 1994;57:763–4. https://doi.org/10.1136/jnnp.57.6.763-a.
Bhidayasiri R, Somers JT, Kim JI, Ramat S, Nayak S, Bokil HS, et al. Ocular oscillations induced by shifts of the direction and depth of visual fixation. Ann Neurol. 2001;49:24–8. https://doi.org/10.1002/1531-8249(200101)49:1<24::aid-ana6>3.0.co;2-t.
Hain TC, Zee DS, Mordes M. Blink-induced saccadic oscillations. Ann Neurol. 1986;19:299–301. https://doi.org/10.1002/ana.410190315.
Brodsky MC, Hunter JS. Positional ocular flutter and thickened optic nerves as sentinel signs of Krabbe disease. J Am Assocr Pediat Ophth Strab. 2011;15:595–7.
Martins AI, Nunes C, Macario MC, Lemos J. Positional ocular flutter associated with middle cerebellar peduncle demyelination. J Neuroophthalmol. 2019;39:117–9. https://doi.org/10.1097/WNO.0000000000000720.
Marti S, Palla A, Straumann D. Gravity dependence of ocular drift in patients with cerebellar downbeat nystagmus. Ann Neurol. 2002;52:712–21. https://doi.org/10.1002/ana.10370.
Choi KD, Oh SY, Park SH, Kim JH, Koo JW, Kim JS. Head-shaking nystagmus in lateral medullary infarction: patterns and possible mechanisms. Neurology. 2007;68:1337–44. https://doi.org/10.1212/01.wnl.0000260224.60943.c2.
Yang Y, Kim JS, Kim S, Kim YK, Kwak YT, Han IW. Cerebellar hypoperfusion during transient global amnesia: an MRI and oculographic study. J Clin Neurol. 2009;5:74–80. https://doi.org/10.3988/jcn.2009.5.2.74.
Ramat S, Das VE, Somers JT, Leigh RJ. Tests of two hypotheses to account for different-sized saccades during disjunctive gaze shifts. Exp Brain Res. 1999;129:500–10. https://doi.org/10.1007/s002210050920.
Fahey MC, Cremer PD, Aw ST, Millist L, Todd MJ, White OB, et al. Vestibular, saccadic and fixation abnormalities in genetically confirmed Friedreich ataxia. Brain. 2008;131:1035–45. https://doi.org/10.1093/brain/awm323.
Shindo K, Onohara A, Hata T, Kobayashi F, Nagasaka K, Nagasaka T, et al. Opsoclonus-myoclonus syndrome associated with multiple system atrophy. Cerebellum Ataxias. 2014;1:15. https://doi.org/10.1186/s40673-014-0015-6.
Evinger C, Kaneko CR, Fuchs AF. Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J Neurophysiol. 1982;47:827–44. https://doi.org/10.1152/jn.1982.47.5.827.
Munoz DP, Wurtz RH. Fixation cells in monkey superior colliculus. II. Reversible activation and deactivation. J Neurophysiol. 1993;70:576–89. https://doi.org/10.1152/jn.1993.70.2.576.
Shallo-Hoffmann J, Sendler B, Muhlendyck H. Normal square wave jerks in differing age groups. Invest Ophthalmol Vis Sci. 1990;31:1649–52.
Ohtsuka K, Mukuno K, Ukai K, Ishikawa S. The origin of square wave jerks: conditions of fixation and microsaccades. Jpn J Ophthalmol. 1986;30:209–15.
Shaikh AG, Miura K, Optican LM, Ramat S, Leigh RJ, Zee DS. A new familial disease of saccadic oscillations and limb tremor provides clues to mechanisms of common tremor disorders. Brain. 2007;130:3020–31. https://doi.org/10.1093/brain/awm240.
Guitton D, Mandl G. A comparison between saccades and quick phases of vestibular nystagmus in the cat. Vis Res. 1980;20:865–73.
Curthoys IS. Generation of the quick phase of horizontal vestibular nystagmus. Exp Brain Res. 2002;143:397–405. https://doi.org/10.1007/s00221-002-1022-z.
Ohki Y, Shimazu H, Suzuki I. Excitatory input to burst neurons from the labyrinth and its mediating pathway in the cat: location and functional characteristics of burster-driving neurons. Exp Brain Res. 1988;72:457–72.
Langer TP, Kaneko CR. Brainstem afferents to the oculomotor omnipause neurons in monkey. J Comp Neurol. 1990;295:413–27. https://doi.org/10.1002/cne.902950306.
McCormick DA, Pape HC. Properties of a hyperpolarization-activated cation current and its role in rhythmic oscillation in thalamic relay neurons. J Physiol. 1990;431:291–318. https://doi.org/10.1113/jphysiol.1990.sp018331.
Shaikh AG, Hain TC, Zee DS. Oculomotor disorders in adult-onset Still’s disease. J Neurol. 2010;257:136–8. https://doi.org/10.1007/s00415-009-5308-y.
Markakis I, Alexiou E, Xifaras M, Gekas G, Rombos A. Opsoclonus-myoclonus-ataxia syndrome with autoantibodies to glutamic acid decarboxylase. Clin Neurol Neurosurg. 2008;110:619–21. https://doi.org/10.1016/j.clineuro.2008.03.005.
Funding
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science and ICT (NRF-2017R1C1B1008582).
Author information
Authors and Affiliations
Contributions
Dr. J.Y. Lee analyzed and interpreted the data and wrote the manuscript. Drs. H.J. Kim and H.J. Oh analyzed and interpreted the data and revised the manuscript. Dr. J.Y. Choi designed and conceptualized the study, interpreted the data, and revised the manuscript. Dr. J. S Kim interpreted the data and critically revised the manuscript.
Corresponding authors
Ethics declarations
Conflict of Interest
Dr. J.S. Kim serves as an associate editor of Frontiers in Neuro-otology and on the editorial boards of the Journal of Korean Society of Clinical Neurophysiology, the Journal of Clinical Neurology, Frontiers in Neuro-ophthalmology, the Journal of Neuro-ophthalmology, the Journal of Vestibular Research, and the Journal of Neurology, and Medicine. Others have no conflicts of interest to disclose.
Ethical Standard
This study followed the tenets of the Declaration of Helsinki and was performed according to the guidelines of the Institutional Review Board of Seoul National University Bundang Hospital (B-1109/135-106).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
(MP4 61,462 kb)
(MP4 107,753 kb)
(MP4 100,614 kb)
Rights and permissions
About this article
Cite this article
Lee, JY., Kwon, E., Kim, HJ. et al. Opsoclonus Following Downbeat Nystagmus in Absence of Visual Fixation in Multiple System Atrophy: Modulation and Mechanisms. Cerebellum 20, 724–733 (2021). https://doi.org/10.1007/s12311-019-01090-w
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12311-019-01090-w