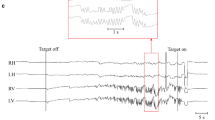
Abstract
Patients with Wernicke’s encephalopathy (WE) often have unusual patterns of vertical nystagmus. Initially there is often a spontaneous upbeating nystagmus that may change to downbeat nystagmus with a change in the direction of gaze, convergence or with vestibular stimuli. Patients also often show a profound loss of the horizontal but not the vertical vestibulo-ocular reflex (VOR). Furthermore, the acute upbeat nystagmus may change to a chronic downbeat nystagmus. We present hypotheses for these features based on (1) the location of vertical gaze-holding networks near the area postrema of the dorsomedial medulla where the blood–brain barrier is located, which we suggest becomes compromised in WE, (2) the location of the vestibular nuclei in the brainstem, medially for the horizontal VOR, and laterally for the vertical VOR, (3) neuronal circuits differ in susceptibility to and in the ability to recover from thiamine deficiency, and (4) impaired processing of otolith information in WE, normally used to modulate translational vestibulo-ocular reflexes, leads to some of the characteristics of the spontaneous vertical nystagmus including the peculiar reversal in its direction with a change in gaze or convergence.



Similar content being viewed by others
References
Kattah JC, Tehrani AS, du Lac S, Newman-Toker DE, Zee DS (2018) Conversion of upbeat to downbeat nystagmus in Wernicke encephalopathy. Neurology 91:790–796
Choi KD, Oh SY, Kim HJ, Kim JS (2007) The vestibulo-ocular reflexes during head impulse in Wernicke's encephalopathy. J Neurol Neurosurg Psychiatry 78:1161–1162
Kattah JC, Guede C, Hassanzadeh B (2018) The medial vestibular nuclei, a vulnerable target in thiamine deficiency. J Neurol 265:213–215
Yoon W (2017) Changing vertical nystagmus in the opposite direction: is the transition from upbeat to downbeat nystagmus a diagnostic clue for Wernicke's encephalopathy? J Neurol Dis 5:370–374
Buttner-Ennever JA, Horn AK (1996) Pathways from cell groups of the paramedian tracts to the floccular region. Ann N Y Acad Sci 781:532–540
Nakamagoe K, Iwamoto Y, Yoshida K (2000) Evidence for brainstem structures participating in oculomotor integration. Science 288:857–859
McEntee WJ (1997) Wernicke's encephalopathy: an excitotoxicity hypothesis. Metab Brain Dis 12:183–192
Harata N, Iwasaki Y (1995) Evidence for early blood-brain barrier breakdown in experimental thiamine deficiency in the mouse. Metab Brain Dis 10:159–174
Nardone R, Holler Y, Storti M et al. (2013) Thiamine deficiency induced neurochemical, neuroanatomical, and neuropsychological alterations: a reappraisal. Sci World J 2013:309143. https://doi.org/10.1155/2013/309143
Jankowska-Kulawy A, Bielarczyk H, Pawelczyk T, Wroblewska M, Szutowicz A (2010) Acetyl-CoA and acetylcholine metabolism in nerve terminal compartment of thiamine deficient rat brain. J Neurochem 115:333–342
Patel VR, Zee DS (2015) The cerebellum in eye movement control: nystagmus, coordinate frames and disconjugacy. Eye (Lond) 29:191–195
Zee DS, Yamazaki A, Butler PH, Gucer G (1981) Effects of ablation of flocculus and paraflocculus of eye movements in primate. J Neurophysiol 46:878–899
Stein B, Carpente MB (1967) Central projections of portions of the vestibular ganglia innervating specific parts of the labyrinth in the rhesus monkey. Am J Anat 120:281–318
Buttner-Ennever JA (1999) A review of otolith pathways to brainstem and cerebellum. Ann N Y Acad Sci 871:51–64
Laurens J, Meng H, Angelaki DE (2013) Computation of linear acceleration through an internal model in the macaque cerebellum. Nat Neurosci 16:1701–1708
Marti S, Straumann D, Buttner U, Glasauer S (2008) A model-based theory on the origin of downbeat nystagmus. Exp Brain Res 188:613–631
Walker MF, Tian J, Shan X, Tamargo RJ, Ying H, Zee DS (2008) Lesions of the cerebellar nodulus and uvula impair downward pursuit. J Neurophysiol 100:1813–1823
Walker MF, Tian J, Shan X, Ying H, Tamargo RJ, Zee DS (2009) Enhancement of the bias component of downbeat nystagmus after lesions of the nodulus and uvula. Ann N Y Acad Sci 1164:482–485
Leigh J, Zee, DS (2015) The neurology of eye movements. Oxford University Press, New York
Helmchen C, Glasauer S, Sprenger A (2013) Inverse eye position dependency of downbeat nystagmus in midline medullary lesion. J Neurol 260:2908–2910
FitzGibbon EJ, Calvert PC, Dieterich M, Brandt T, Zee DS (1996) Torsional nystagmus during vertical pursuit. J Neurophthalmol 16:79–90
Tian J, Zee DS, Walker MF (2007) Rotational and translational optokinetic nystagmus have different kinematics. Vis Res 47:1003–1010
Straumann D, Zee DS, Solomon D (2000) Three-dimensional kinematics of ocular drift in humans with cerebellar atrophy. J Neurophysiol 83:1125–1140
Pierrot-Deseilligny C (2005) Vertical nystagmus: clinical facts and hypotheses. Brain 128:1237–1246
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare that they have no competing interests.
Ethical approval
All experiments followed the tenets of the Declaration of Helsinki and this study was approved by the Institutional Review Board.
Informed consent
The patient gave written consent to use this video for publication.
Additional information
This manuscript is part of a supplement sponsored by the German Federal Ministry of Education and Research within the funding initiative for integrated research and treatment centers.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Baseline and Follow-up Video recording of case 3 (table 1). Baseline: The first video section was obtained three months after the diagnosis of WE, he has a robust UBN in straight-ahead gaze. In lateral gaze note DBN mixed with a horizontal component, right beat in right gaze and left beat in left gaze. Follow-up: The second Video section was obtained 10 weeks after the first recording: No nystagmus is present in straight-ahead gaze though with direct ophthalmoscopy and slit lamp examination there was a small-amplitude UBN. In lateral gaze he has a robust DBN (MP4 208212 kb)
Rights and permissions
About this article
Cite this article
Kattah, J.C., McClelland, C. & Zee, D.S. Vertical nystagmus in Wernicke’s encephalopathy: pathogenesis and role of central processing of information from the otoliths. J Neurol 266 (Suppl 1), 139–145 (2019). https://doi.org/10.1007/s00415-019-09326-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00415-019-09326-9