Abstract
The human saccadic system is potentially unstable and may oscillate if the burst neurons, which generate saccades, are not inhibited by omnipause neurons. A previous study showed that combined saccade vergence movements can evoke oscillations in normal subjects. We set out to determine: 1) whether similar oscillations can be recorded during other paradigms associated with inhibition of omnipause neurons; 2) whether lesions of the fastigial nuclei disrupt such oscillations; and 3) whether such oscillations can be reproduced using a model based on the coupling of excitatory and inhibitory burst neurons. We recorded saccadic oscillations during vergence movements, combined saccade-vergence movements, vertical saccades, pure vergence and blinks in three normal subjects, and in a patient with saccadic hypermetria due to a surgical lesion affecting both fastigial nuclei. During combined saccade-vergence, normal subjects and the cerebellar patient developed small-amplitude (0.1–0.5°), high-frequency (27–35 Hz), conjugate horizontal saccadic oscillations. Oscillations of a similar amplitude and frequency occurred during blinks, pure vergence and vertical saccades. One normal subject could generate saccadic oscillations voluntarily (~0.7° amplitude, 25 Hz) during sustained convergence. Previous models proposed that high-frequency eye oscillations produced by the saccadic system (saccadic oscillations), occur because of a delay in a negative feedback loop around high-gain, excitatory burst neurons in the brainstem. The feedback included the cerebellar fastigial nuclei. We propose another model that accounts for saccadic oscillations based on 1) coupling of excitatory and inhibitory burst neurons in the brainstem and 2) the hypothesis that burst neurons show post-inhibitory rebound discharge. When omnipause neurons are inhibited (as during saccades, saccade-vergence movements and blinks), this new model simulates oscillations with amplitudes and frequencies comparable to those in normal human subjects. The finding of saccadic oscillations in the cerebellar patient is compatible with the new model but not with the recent models including the fastigial nuclei in the classic negative-feedback loop model. Our model proposes a novel mechanism for generating oscillations in the oculomotor system and perhaps in other motor systems too.












Similar content being viewed by others
References
Aizenman CD, Linden DJ (1999) Regulation of the rebound depolarization and spontaneous firing patterns of deep nuclear neurons in slices of rat cerebellum. J Neurophysiol 82:1697–1709
Ashe J, Hain TC, Zee DS, Schatz NJ (1991) Microsaccadic flutter. Brain 114:461–472
Bergamin O, Zee DS, Roberts DC, Landau K, Lasker AG, Straumann D (2001) Three-dimensional Hess screen test with binocular dual search coils in a three-field magnetic system. Invest Ophthalmol Vis Sci 42:660–667
Bhidayasiri R, Somers JT, Kim JI, Ramat S, Nayak S, Bokil HS, Leigh RJ (2001) Ocular oscillations induced by shifts of the direction and depth of visual fixation. Ann Neurol 49:24–28
Busettini C, Mays LE (2003) Pontine omnipause activity during conjugate and disconjugate eye movements in macaques. J Neurophysiol 90:3838–3853
Büttner-Ennever JA, Büttner U (1992) Neuroanatomy of the ocular motor pathways. Baillieres Clin Neurol 1:263–287
Büttner-Ennever JA, Cohen B, Pause M, Fries W (1988) Raphe nucleus of the pons containing omnipause neurons of the oculomotor system in the monkey, and its homologue in man. J Comp Neurol 267:307–321
Chun KS, Robinson DA (1978) A model of quick phase generation in the vestibuloocular reflex. Biol Cybern 28:209–221
Collewijn H, van der SJ, Steinman RM (1985) Human eye movements associated with blinks and prolonged eyelid closure. J Neurophysiol 54:11–27
Dean P (1995) Modelling the role of the cerebellar fastigial nuclei in producing accurate saccades: the importance of burst timing. Neuroscience 68:1059–1077
Duvernoy HM (1995) The human brain stem and the cerebellum. Springer, New York
Efron B, Tibshirani RJ (1993) An introduction to the bootstrap. Chapman and Hall, New York
Enderle JD, Engelken EJ (1995) Simulation of oculomotor post-inhibitory rebound burst firing using a Hodgkin-Huxley model of a neuron. Biomed Sci Instrum 31:53–58
Evinger C, Kaneko CR, Fuchs AF (1982) Activity of omnipause neurons in alert cats during saccadic eye movements and visual stimuli. J Neurophysiol 47:827–844
Hain TC, Zee DS, Mordes M (1986) Blink-induced saccadic oscillations. Ann Neurol 19:299–301
Hepp K, Henn V, Vilis T, Cohen B (1989) Brainstem regions related to saccade generation. Rev Oculomot Res 3:105–212
Horn AK, Büttner-Ennever JA, Suzuki Y, Henn V (1995) Histological identification of premotor neurons for horizontal saccades in monkey and man by parvalbumin immunostaining. J Comp Neurol 359:350–363
Hotson JR (1984) Convergence-initiated voluntary flutter: a normal intrinsic capability in man. Brain Res 294:299–304
Huguenard JR (1998) Low-voltage-activated (T-type) calcium-channel genes identified. Trends Neurosci 21:451–452
Huntsman MM, Porcello DM, Homanics GE, DeLorey TM, Huguenard JR (1999) Reciprocal inhibitory connections and network synchrony in the mammalian thalamus. Science 283:541–543
Jacobsen RB, Ulrich D, Huguenard JR (2001) GABA(B) and NMDA receptors contribute to spindle-like oscillations in rat thalamus in vitro. J Neurophysiol 86:1365–1375
Jurgens R, Becker W, Kornhuber HH (1981) Natural and drug-induced variations of velocity and duration of human saccadic eye movements: evidence for a control of the neural pulse generator by local feedback. Biol Cybern 39:87–96
Keller EL (1974) Participation of medial pontine reticular formation in eye movement generation in monkey. J Neurophysiol 37:316–332
Lefèvre P, Quaia C, Optican LM (1998) Distributed model of control of saccades by superior colliculus and cerebellum. Neural Netw 11:1175–1190
Leigh RJ, Zee DS (1999) The neurology of eye movements. Oxford University Press
Mays LE, Gamlin PD (1995) Neuronal circuitry controlling the near response. Curr Opin Neurobiol 5:763–768
Mays LE, Morrisse DW (1995) Electrical stimulation of the pontine omnipause area inhibits eye blink. J Am Optom Assoc 66:419–422
Optican LM, Quaia C (2002) Distributed model of collicular and cerebellar function during saccades. Ann N Y Acad Sci 956:164–177
Perez-Reyes E (2003) Molecular physiology of low-voltage-activated t-type calcium channels. Physiol Rev 83:117–161
Quaia C, Optican LM (1997) Model with distributed vectorial premotor bursters accounts for the component stretching of oblique saccades. J Neurophysiol 78:1120–1134
Quaia C, Lefevre P, Optican LM (1999) Model of the control of saccades by superior colliculus and cerebellum. J Neurophysiol 82:999–1018
Ramat S, Somers JT, Das VE, Leigh RJ (1999) Conjugate ocular oscillations during shifts of the direction and depth of visual fixation. Invest Ophthalmol Vis Sci 40:1681–1686
Roberts A, Tunstall MJ (1990) Mutual re-excitation with post-inhibitory rebound: a simulation study on the mechanisms for locomotor rhythm generation in the spinal cord of xenopus embryos. Eur J Neurosci 2:11–23
Robinson DA (1964) The mechanics of human saccadic eye movement. J Physiol (London) 174:245–264
Robinson DA (1975) Oculomotor control signals. In: Lennerstrand G, Bach-y-Rita P (eds) Basic mechanisms of ocular motility and their clinical implications. Pergamon Press, Oxford, pp 337–374
Robinson FR, Fuchs AF (2001) The role of the cerebellum in voluntary eye movements. Annu Rev Neurosci 24:981–1004
Robinson DA, Keller EL (1972) The behavior of eye movement motoneurons in the alert monkey. Bibl Ophthalmol 82:7–16
Robinson FR, Straube A, Fuchs AF (1993) Role of the caudal fastigial nucleus in saccade generation. II. Effects of muscimol inactivation. J Neurophysiol 70:1741–1758
Rottach KG, Das VE, Wohlgemuth W, Zivotofsky AZ, Leigh RJ (1998) Properties of horizontal saccades accompanied by blinks. J Neurophysiol 79:2895–2902
Scudder CA (1988) A new local feedback model of the saccadic burst generator. J Neurophysiol 59:1455–1475
Scudder CA, Fuchs AF, Langer TP (1988) Characteristics and functional identification of saccadic inhibitory burst neurons in the alert monkey. J Neurophysiol 59:1430–1454
Scudder CA, Kaneko CS, Fuchs AF (2002) The brainstem burst generator for saccadic eye movements. A modern synthesis. Exp Brain Res 142:439–462
Sekirnjak C, du Lac S (2002) Intrinsic firing dynamics of vestibular nucleus neurons. J Neurosci 22:2083–2095
Shults WT, Stark L, Hoyt WF, Ochs AL (1977) Normal saccadic structure of voluntary nystagmus. Arch Ophthalmol 95:1399–1404
Soetedjo R, Kaneko CR, Fuchs AF (2002) Evidence that the superior colliculus participates in the feedback control of saccadic eye movements. J Neurophysiol 87:679–695
Sohal VS, Huntsman MM, Huguenard JR (2000) Reciprocal inhibitory connections regulate the spatiotemporal properties of intrathalamic oscillations. J Neurosci 20:1735–1745
Sparks DL (2002) The brainstem control of saccadic eye movements. Nat Rev Neurosci 3:952–964
Strassman A, Highstein SM, McCrea RA (1986a) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. I. Excitatory burst neurons. J Comp Neurol 249:337–357
Strassman A, Highstein SM, McCrea RA (1986b) Anatomy and physiology of saccadic burst neurons in the alert squirrel monkey. II. Inhibitory burst neurons. J Comp Neurol 249:358–380
Sylvestre PA, Galiana HL, Cullen KE (2002) Conjugate and vergence oscillations during saccades and gaze shifts: implications for integrated control of binocular movement. J Neurophysiol 87:257–272
Van Gisbergen JA, Robinson DA, Gielen S (1981) A quantitative analysis of generation of saccadic eye movements by burst neurons. J Neurophysiol 45:417–442
Wong AM, Musallam S, Tomlinson RD, Shannon P, Sharpe JA (2001) Opsoclonus in three dimensions: oculographic, neuropathologic and modelling correlates. J Neurol Sci 189:71–81
Yee RD, Spiegel PH, Yamada T, Abel LA, Suzuki DA, Zee DS (1994) Voluntary saccadic oscillations, resembling ocular flutter and opsoclonus. J Neuroophthalmol 14:95–101
Zee DS, Hain TC (1992) Clinical implications of otolith-ocular reflexes. Am J Otol 13:152–157
Zee DS, Robinson DA (1979) A hypothetical explanation of saccadic oscillations. Ann Neurol 5:405–414
Zee DS, Fitzgibbon EJ, Optican LM (1992) Saccade-vergence interactions in humans. J Neurophysiol 68:1624–1641
Acknowledgements
Supported by USPHS grant EY06717, the Office of Research and Development, Medical Research Service, Department of Veterans Affairs, and the Evenor Armington Fund (to R.J. Leigh), USPHS grant EY01849 to D.S. Zee. David Linden provided helpful discussion. Dr. Stefano Ramat was supported by the Robert M. and Annetta J. Coffelt Endowment for PSP research. The authors are grateful to Jeffrey Somers for assistance with experiments.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
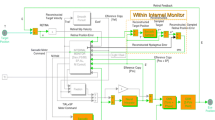
The model of coupled burst neurons was simulated in Simulink/Matlab. The structure of the model included OPN and two EBN/IBN pairs, connected as shown in Fig. 7. Individual neurons contained an adaptation element (as in Fig. 8a) and a synaptic delay (τ). Values for the cross-coupling coefficients and other dynamic properties are given in Table 2. The final common path (i.e., neural integrator and orbital tissues) was modeled with an integrator and one uncompensated, single-pole filter (time constant Teye).
The medium lead burst neurons were represented by bilateral pairs of inhibitory and excitatory burst neurons. Both EBN and IBN were modeled like the OPN, except that a soft-saturating nonlinearity was added to the output. The equation for this element comes from Zee and Robinson (1979):
This element was added to reduce the harmonic distortion in the saccadic oscillations that was present when a hard saturation element was used. In this equation the input variable e represents motor error for the Robinson model, whereas in our model e represents an input in terms of the frequency of discharge rate, which is related to eye velocity. The values for the parameters in these equations were therefore derived from those in the original model and scaled to reflect the different input variables (cf. Table 2). The EBN and IBN were reciprocally coupled across the midline. EBN projected to ipsilateral IBN with a gain of 1.0. IBN projected to contralateral EBN with a gain of 1.0 and to contralateral IBN with a gain of either ibnLR (Left to Right side) or ibnRL (Right to Left side). These gain values were not perfectly symmetric, to ensure that the system would start oscillating as soon as the OPN shut off. When equal gains were used, the system was quasi-stable, and would only begin to oscillate after a few hundred milliseconds.
Rights and permissions
About this article
Cite this article
Ramat, S., Leigh, R.J., Zee, D.S. et al. Ocular oscillations generated by coupling of brainstem excitatory and inhibitory saccadic burst neurons. Exp Brain Res 160, 89–106 (2005). https://doi.org/10.1007/s00221-004-1989-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-004-1989-8