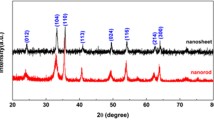
Abstract
In the quest to enhance the efficiency of sodium-ion batteries, the dynamics of solid electrolyte interphase (SEI) formation are of paramount importance. The SEI layer’s integrity is integral to the charge–discharge efficiency and the overall longevity of the battery. Herein, a novel two-dimensional Ti3C2 fragments enmeshed on iron-nitrogen-carbon (Fe-N-C) nanosheets (Ti3C2/Fe-N-C) has been synthesized. This electrode features a matrix which has been shown to expedite SEI layer formation through the facilitation of selective anion adsorption, thus augmenting battery performance. Density functional theory calculation reveals that the SEI evolution energy of NaPF6 at the Ti3C2/Fe-N-C interface is 0.81 eV, significantly lower than the Ti3C2 (1.23 eV). This process is driven by the electron transportation from Ti3C2 to Fe-N-C substrate, facilitated by their work-function difference, leading to the formation of ferromagnetic Fe species, which possesses Fe 3d \(\mathrm{d}_{xz}\mathrm{d}_{yz}\mathrm{d}_{z^{2}}\) orbitals and undergoes hybridization with the π and σ orbitals of NaF, creating a key intermediate during charging. This process diminishes the antibonding energy and attenuates the orbital interaction with NaF, thus reducing the activation energy and improving the SEI formation reaction kinetics. Consequently, it leads to the creation of multi-interface SEI characterized by high-throughput ion transport and an efficient reaction network.

Similar content being viewed by others
References
Tarascon, J. M.; Armand, M. Issues and challenges facing rechargeable lithium batteries. Nature 2001, 414, 359–367.
Armand, M.; Tarascon, J. M. Building better batteries. Nature 2008, 451, 652–657.
Liang, Y. L.; Dong, H.; Aurbach, D.; Yao, Y. Current status and future directions of multivalent metal-ion batteries. Nat. Energy 2020, 5, 646–656.
Luo, W.; Shen, F.; Bommier, C.; Zhu, H. L.; Ji, X. L.; Hu, L. B. Na-ion battery anodes: Materials and electrochemistry. Acc. Chem. Res. 2016, 49, 231–240.
Li, Y.; Zhang, J. W.; Chen, Q. G.; Xia, X. H.; Chen, M. H. Emerging of heterostructure materials in energy storage: A review. Adv. Mater. 2021, 33, 2100855.
Huang, Z. J.; Lai, J. C.; Liao, S. L.; Yu, Z. A.; Chen, Y. L.; Yu, W. L.; Gong, H. X.; Gao, X.; Yang, Y. F.; Qin, J. et al. A salt-philic, solvent-phobic interfacial coating design for lithium metal electrodes. Nat. Energy 2023, 8, 577–585.
Gao, Y.; Yan, Z. F.; Gray, J. L.; He, X.; Wang, D. W.; Chen, T. H.; Huang, Q. Q.; Li, Y. C.; Wang, H. Y.; Kim, S. H. et al. Polymer–inorganic solid–electrolyte interphase for stable lithium metal batteries under lean electrolyte conditions. Nat. Mater. 2019, 18, 384–389.
Wu, H.; Chan, G.; Choi, J. W.; Ryu, I.; Yao, Y.; McDowell, M. T.; Lee, S. W.; Jackson, A.; Yang, Y.; Hu, L. et al. Stable cycling of double-walled silicon nanotube battery anodes through solid-electrolyte interphase control. Nat. Nanotechnol. 2012, 7, 310–315.
Deng, T.; Ji, X.; Zou, L. F.; Chiekezi, O.; Cao, L. S.; Fan, X. L.; Adebisi, T. R.; Chang, H. J.; Wang, H.; Li, B. et al. Interfacial-engineering-enabled practical low-temperature sodium metal battery. Nat. Nanotechnol. 2022, 17, 269–277.
Li, Y.; Liu, M. Q.; Feng, X.; Li, Y.; Wu, F.; Bai, Y.; Wu, C. How can the electrode influence the formation of the solid electrolyte interface. ACS Energy Lett. 2021, 6, 3307–3320.
Wang, H. P.; Zhu, C. L.; Liu, J. D.; Qi, S. H.; Wu, M. G.; Huang, J. D.; Wu, D. X.; Ma, J. M. Formation of NaF-rich solid electrolyte interphase on Na Anode through additive-induced anion-enriched structure of Na+ solvation. Angew. Chem., Int. Ed. 2022, 61, e202208506.
Gu, T.; Chen, L. K.; Huang, Y. F.; Ma, J. B.; Shi, P. R.; Biao, J.; Liu, M.; Lv, W.; He, Y. B. Engineering ferroelectric interlayer between Li1.3Al0.3Ti1.7(PO4)3 and lithium metal for stable solid-state batteries operating at room temperature. Energy Environ. Mater. 2023, 6, e12531.
Zhu, C. L.; Wu, D. X.; Wang, Z. S.; Wang, H. P.; Liu, J. D.; Guo, K. L.; Liu, Q. H.; Ma, J. M. Optimizing NaF-rich solid electrolyte interphase for stabilizing sodium metal batteries by electrolyte additive. Adv. Funct. Mater. 2024, 34, 2214195.
Xu, M. Y.; Li, Y.; Ihsan-Ul-Haq, M.; Mubarak, N.; Liu, Z. J.; Wu, J. X.; Luo, Z. T.; Kim, J. K. NaF-rich solid electrolyte interphase for dendrite-free sodium metal batteries. Energy Storage Mater. 2022, 44, 477–486.
Wang, Y. L.; Liu, F. M.; Fan, G. L.; Qiu, X. G.; Liu, J. D.; Yan, Z. H.; Zhang, K.; Cheng, F. Y.; Chen, J. Electroless formation of a fluorinated Li/Na hybrid interphase for robust lithium anodes. J. Am. Chem. Soc. 2021, 143, 2829–2837.
Liu, P. C.; Xiao, P.; Lu, M.; Wang, H.; Jin, N.; Lin, Z. F. Lithium storage properties of Ti3C2Tx (Tx = F, Cl, Br) MXenes. Chin. Chem. Lett. 2023, 34, 107426.
Xia, H. C.; Qu, G.; Yin, H. B.; Zhang, J. A. Atomically dispersed metal active centers as a chemically tunable platform for energy storage devices. J. Mater. Chem. A 2020, 8, 15358–15372.
Man, Q. Y.; An, Y. L.; Liu, C. K.; Shen, H. T.; Xiong, S. L.; Feng, J. K. Interfacial design of silicon/carbon anodes for rechargeable batteries: A review. J. Energy Chem. 2023, 76, 576–600.
Wang, J.; Du, C. F.; Xue, Y.; Tan, X.; Kang, J.; Gao, Y.; Yu, H.; Yan, Q. MXenes as a versatile platform for reactive surface modification and superior sodium-ion storages. Exploration 2021, 1, 20210024.
He, Y. H.; Liu, S. W.; Priest, C.; Shi, Q. R.; Wu, G. Atomically dispersed metal-nitrogen-carbon catalysts for fuel cells: Advances in catalyst design, electrode performance, and durability improvement. Chem. Soc. Rev. 2020, 49, 3484–3524.
Zhu, Y. P.; Guo, C. X.; Zheng, Y.; Qiao, S. Z. Surface and interface engineering of noble-metal-free electrocatalysts for efficient energy conversion processes. Acc. Chem. Res. 2017, 50, 915–923.
Xia, H. C.; Zan, L. X.; Yuan, P. F.; Qu, G.; Dong, H. L.; Wei, Y. F.; Yu, Y.; Wei, Z. Y.; Yan, W. F.; Hu, J. S. et al. Evolution of stabilized 1T-MoS2 by atomic-interface engineering of 2H-MoS2/Fe-Nx towards enhanced sodium ion storage. Angew. Chem., Int. Ed. 2023, 62, e202218282.
Bai, X.; Han, J. Y.; Niu, X. D.; Guan, J. Q. The d-orbital regulation of isolated manganese sites for enhanced oxygen evolution. Nano Res. 2023, 16, 10796–10802.
Luo, F.; Roy, A.; Sougrati, M. T.; Khan, A.; Cullen, D. A.; Wang, X. L.; Primbs, M.; Zitolo, A.; Jaouen, F.; Strasser, P. Structural and reactivity effects of secondary metal doping into iron-nitrogen-carbon catalysts for oxygen electroreduction. J. Am. Chem. Soc. 2023, 145, 14737–14747.
Zhu, C. Z.; Li, H.; Fu, S. F.; Du, D.; Lin, Y. H. Highly efficient nonprecious metal catalysts towards oxygen reduction reaction based on three-dimensional porous carbon nanostructures. Chem. Soc. Rev. 2016, 45, 517–531.
Yin, H. B.; Xia, H. C.; Zhao, S. Y.; Li, K. X.; Zhang, J. N.; Mu, S. C. Atomic level dispersed metal-nitrogen-carbon catalyst toward oxygen reduction reaction: Synthesis strategies and chemical environmental regulation. Energy Environ. Mater. 2021, 4, 5–18.
Wang, Y. X.; Cui, X. Z.; Peng, L. W.; Li, L. L.; Qiao, J. L.; Huang, H. T.; Shi, J. L. Metal-nitrogen-carbon catalysts of specifically coordinated configurations toward typical electrochemical redox reactions. Adv. Mater. 2021, 33, 2100997.
Zhu, C.; Yang, J.; Zhang, J.; Wang, X.; Gao, Y.; Wang, D.; Pan, H. Single-atom materials: The application in energy conversion. Interdiscip. Mater. 2024, 3, 74–86.
Shi, S. Q.; Lu, P.; Liu, Z. Y.; Qi, Y.; Hector, L. G. Jr; Li, H.; Harris, S. J. Direct calculation of Li-ion transport in the solid electrolyte interphase. J. Am. Chem. Soc. 2012, 134, 15476–15487.
Augustyn, V.; Come, J.; Lowe, M. A.; Kim, J. W.; Taberna, P. L.; Tolbert, S. H.; Abruña, H. D.; Simon, P.; Dunn, B. High-rate electrochemical energy storage through Li+ intercalation pseudocapacitance. Nat. Mater. 2013, 12, 518–522.
Wang, J.; Li, Y. S.; Liu, P.; Wang, F.; Yao, Q. R.; Zou, Y. J.; Zhou, H. Y.; Balogun, M. S.; Deng, J. Q. Green large-scale production of N/O-dual doping hard carbon derived from bagasse as high-performance anodes for sodium-ion batteries. J. Cent. South Univ. 2021, 28, 361–369.
Ramasubramanian, A.; Yurkiv, V.; Foroozan, T.; Ragone, M.; Shahbazian-Yassar, R.; Mashayek, F. Lithium diffusion mechanism through solid-electrolyte interphase in rechargeable lithium batteries. J. Phys. Chem. C 2019, 123, 10237–10245.
Gao, Y.; Zhang, B. Probing the mechanically stable solid electrolyte interphase and the implications in design strategies. Adv. Mater. 2023, 35, 2205421.
Zhang, M. H.; Kitchaev, D. A.; Lebens-Higgins, Z.; Vinckeviciute, J.; Zuba, M.; Reeves, P. J.; Grey, C. P.; Whittingham, M. S.; Piper, L. F. J.; Van der Ven, A. et al. Pushing the limit of 3d transition metal-based layered oxides that use both cation and anion redox for energy storage. Nat. Rev. Mater. 2022, 7, 522–540.
Meng, Y. S.; Srinivasan, V.; Xu, K. Designing better electrolytes. Science 2022, 378, eabq3750.
Feng, X. Y.; Fang, H.; Wu, N.; Liu, P. C.; Jena, P.; Nanda, J.; Mitlin, D. Review of modification strategies in emerging inorganic solid-state electrolytes for lithium, sodium, and potassium batteries. Joule 2022, 6, 543–587.
Miao, X.; Guan, S. D.; Ma, C.; Li, L. L.; Nan, C. W. Role of interfaces in solid-state batteries. Adv. Mater. 2023, 35, 2206402.
Peng, M. Q.; Shin, K.; Jiang, L. X.; Jin, Y.; Zeng, K.; Zhou, X. L.; Tang, Y. B. Alloy-type anodes for high-performance rechargeable batteries. Angew. Chem., Int. Ed. 2022, 61, e202206770.
Xia, H. C.; Zan, L. X.; Qu, G.; Tu, Y. C.; Dong, H. L.; Wei, Y. F.; Zhu, K. X.; Yu, Y.; Hu, Y. F.; Deng, D. H. et al. Evolution of a solid electrolyte interphase enabled by FeNx/C catalysts for sodium-ion storage. Energy Environ. Sci. 2022, 15, 771–779.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 32, 2003300.
Gan, T.; Wang, D. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res. 2023, 17, 18–38.
Wei, Y. F.; Xia, H. C.; Yan, W. F.; Zhang, J. N. Recent processing of interaction mechanisms of single metallic atom/clusters in energy electrocatalysis. Energy Mater. 2023, 3, 300033.
Zhang, Y. Q.; Yang, J.; Ge, R. Y.; Zhang, J. J.; Cairney, J. M.; Li, Y.; Zhu, M. Y.; Li, S. A.; Li, W. X. The effect of coordination environment on the activity and selectivity of single-atom catalysts. Coord. Chem. Rev. 2022, 461, 214493.
Wigner, E. On the interaction of electrons in metals. Phys. Rev. 1934, 46, 1002–1011.
Feder, R. Spin polarization in low-energy electron diffraction from W(001). Phys. Rev. Lett. 1976, 36, 598–600.
Wang, Y. J.; Cheng, W. Z.; Yuan, P. F.; Yang, G. G.; Mu, S. C.; Liang, J. L.; Xia, H. C.; Guo, K.; Liu, M. L.; Zhao, S. Y. et al. Boosting nitrogen reduction to ammonia on FeN4 sites by atomic spin regulation. Adv. Sci. 2021, 8, 2102915.
Oliver, G. L.; Perdew, J. P. Spin-density gradient expansion for the kinetic energy. Phys. Rev. A 1979, 20, 397–403.
Cole, L. A.; Perdew, J. P. Calculated electron affinities of the elements. Phys. Rev. A 1982, 25, 1265–1271.
Liu, X. C.; Choi, M. S.; Hwang, E.; Yoo, W. J.; Sun, J. Fermi level pinning dependent 2D semiconductor devices: Challenges and prospects. Adv. Mater. 2022, 34, 2108425.
Zitolo, A.; Goellner, V.; Armel, V.; Sougrati, M. T.; Mineva, T.; Stievano, L.; Fonda, E.; Jaouen, F. Identification of catalytic sites for oxygen reduction in iron- and nitrogen-doped graphene materials. Nat. Mater. 2015, 14, 937–942.
Choi, C. H.; Choi, W. S.; Kasian, O.; Mechler, A. K.; Sougrati, M. T.; Brüller, S.; Strickland, K.; Jia, Q. Y.; Mukerjee, S.; Mayrhofer, K. J. J. et al. Unraveling the nature of sites active toward hydrogen peroxide reduction in Fe-N-C catalysts. Angew. Chem., Int. Ed. 2017, 56, 8809–8812.
Liu, W. G.; Zhang, L. L.; Liu, X.; Liu, X. Y.; Yang, X. F.; Miao, S.; Wang, W. T.; Wang, A. Q.; Zhang, T. Discriminating catalytically active FeNx species of atomically dispersed Fe-N-C catalyst for selective oxidation of the C–H bond. J. Am. Chem. Soc. 2017, 139, 10790–10798.
Kramm, U. I.; Lefèvre, M.; Larouche, N.; Schmeisser, D.; Dodelet, J. P. Correlations between mass activity and physicochemical properties of Fe/N/C catalysts for the ORR in PEM fuel cell via 57Fe Mossbauer spectroscopy and other techniques. J. Am. Chem. Soc. 2014, 136, 978–985.
Li, J. S.; Zheng, C. Y.; Zhao, E. L.; Mao, J.; Cheng, Y. H.; Liu, H.; Hu, Z. P.; Ling, T. Ferromagnetic ordering correlated strong metal-oxygen hybridization for superior oxygen reduction reaction activity. Proc. Natl. Acad. Sci. USA 2023, 120, e2307901120.
Liu, J. L.; Wang, J.; Xu, C. H.; Jiang, H.; Li, C. Z.; Zhang, L. L.; Lin, J. Y.; Shen, Z. X. Advanced energy storage devices: Basic principles, analytical methods, and rational materials design. Adv. Sci. 2018, 5, 1700322.
Wang, J.; Polleux, J.; Lim, J.; Dunn, B. Pseudocapacitive contributions to electrochemical energy storage in TiO2 (anatase) nanoparticles. J. Phys. Chem. C 2007, 111, 14925–14931.
Augustyn, V.; Simon, P.; Dunn, B. Pseudocapacitive oxide materials for high-rate electrochemical energy storage. Energy Environ. Sci. 2014, 7, 1597–1614.
Barsoukov, E.; Kim, D. H.; Lee, H. S.; Lee, H.; Yakovleva, M.; Gao, Y.; Engel, J. F. Comparison of kinetic properties of LiCoO2 and LiTi0.05Mg0.05Ni0.7Co0.2O2 by impedance spectroscopy. Solid State Ionics 2003, 161, 19–29.
Busche, M. R.; Drossel, T.; Leichtweiss, T.; Weber, D. A.; Falk, M.; Schneider, M.; Reich, M. L.; Sommer, H.; Adelhelm, P.; Janek, J. Dynamic formation of a solid–liquid electrolyte interphase and its consequences for hybrid-battery concepts. Nat. Chem. 2016, 8, 426–434.
Choudhury, S.; Wei, S. Y.; Ozhabes, Y.; Gunceler, D.; Zachman, M. J.; Tu, Z. Y.; Shin, J. H.; Nath, P.; Agrawal, A.; Kourkoutis, L. F. et al. Designing solid–liquid interphases for sodium batteries. Nat. Commun. 2017, 8, 898.
Rui, X. H.; Ding, N.; Liu, J.; Li, C.; Chen, C. H. Analysis of the chemical diffusion coefficient of lithium ions in Li3V2(PO4)3 cathode material. Electrochim. Acta 2010, 55, 2384–2390.
Liu, T. C.; Lin, L. P.; Bi, X. X.; Tian, L. L.; Yang, K.; Liu, J. J.; Li, M. F.; Chen, Z. H.; Lu, J.; Amine, K. et al. In situ quantification of interphasial chemistry in Li-ion battery. Nat. Nanotechnol. 2019, 14, 50–56
Gervillié-Mouravieff, C.; Boussard-Plédel, C.; Huang, J. Q.; Leau, C.; Blanquer, L. A.; Yahia, M. B.; Doublet, M. L.; Boles, S. T.; Zhang, X. H.; Adam, J. L. et al. Unlocking cell chemistry evolution with operando fibre optic infrared spectroscopy in commercial Na(Li)-ion batteries. Nat. Energy 2022, 7, 1157–1169.
Chen, D. C.; Mahmoud, M. A.; Wang, J. H.; Waller, G. H.; Zhao, B. T.; Qu, C.; El-Sayed, M. A.; Liu, M. L. Operando investigation into dynamic evolution of cathode–electrolyte interfaces in a Li-ion battery. Nano Lett. 2019, 19, 2037–2043.
Yin, X. P.; Lu, Z. X.; Wang, J.; Feng, X. C.; Roy, S.; Liu, X. S.; Yang, Y.; Zhao, Y. F.; Zhang, J. J. Enabling fast Na+ transfer kinetics in the whole-voltage-region of hard-carbon anodes for ultrahigh-rate sodium storage. Adv. Mater. 2022, 34, 2109282.
Acknowledgement
This work was supported by the National Natural Science Foundation of China (Nos. U22A20107, 22162026, and 42050203), the Science and Technology Research and Develpoment Program Joint Fund Project of Henan Provincial (No. 222301420001), the Distinguished Young Scholars Innovation Team of Zhengzhou University (No. 32320275), Key Research Projects of University in Henan Province (No. 24A150041), Henan Province Science and Technology Research Projects (No. 242102240106), and Postdoctoral Fellowship Program of CPSF (No. GZC20232382). The authors also gratefully acknowledge the BL14W1 and BL11B at Shanghai Synchrotron Radiation Facilities (SSRF) and beamline 4B9A at Beijing Synchrotron Radiation Facility (BSRF) for the XAFS measurements.
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Xia, H., Zan, L., Dong, H. et al. Work-function effect of Ti3C2/Fe-N-C inducing solid electrolyte interphase evolution for ultra-stable sodium storage. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6693-3
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6693-3