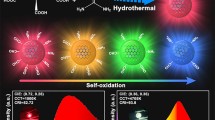
Abstract
Prussian blue (PB), as a promising inorganic electrochromic material (ECM), has been widely used in smart windows, displays, sensors, etc. However, there are still many challenges for PB to achieve high electrochromic performance. Herein, we synthesized nitrogen-doped carbon dots-modified PB film (defined as PB@N-CDs) with a sandwich-like structure by a simple stepwise electrodeposition method. The carbon dots show an obvious advantage in ultrafast electron transfer ability, which can reduce charge loss during the transfer process, improve the electrochemical activity on both sides of PB, and thus facilitate a rapid electrochromic response. Furthermore, the surface of nitrogen-doped carbon dots contains multiple organic functional groups, which widen the movement path of K+ ions under electrostatic adsorption. Impressively, the PB@N-CDs film exhibits a short bleaching/coloring time (0.5/0.9 s) and a superior optical modulation range (78.6%). Particularly, the coloring efficiency has been significantly improved to 137.71 cm2/C (at 700 nm). All of these results open up new avenues for developing high-performance PB-based ECMs and promoting their applications in corresponding electrochromic devices (ECDs) and smart windows.

Similar content being viewed by others
References
Su, L. Y.; Lu, Z. H.; Wei, Y. An all solid-state electrochromic smart window. Chin. Sci. Bull. 1998, 43, 944–947.
Kim, Y.; Han, M. S.; Kim, J.; Kim, E. Electrochromic capacitive windows based on all conjugated polymers for a dual function smart window. Energy Environ. Sci. 2018, 11, 2124–2133.
Kim, H. N.; Cho, S. M.; Ah, C. S.; Song, J.; Ryu, H.; Kim, Y. H.; Kim, T. Y. Electrochromic mirror using viologen-anchored nanoparticles. Mater. Res. Bull. 2016, 82, 16–21.
Yan, C. Y.; Kang, W. B.; Wang, J. X.; Cui, M. Q.; Wang, X.; Foo, C. Y.; Chee, K. J.; Lee, P. S. Stretchable and wearable electrochromic devices. ACS Nano 2014, 8, 316–322.
Gu, C.; Jia, A. B.; Zhang, Y. M.; Zhang, S. X. A. Emerging electrochromic materials and devices for future displays. Chem. Rev. 2022, 122, 14679–14721.
Chen, K.; He, J. Z.; Zhang, D.; You, L. Y.; Li, X. F.; Wang, H. Y.; Mei, J. G. Bioinspired dynamic camouflage from colloidal nanocrystals embedded electrochromics. Nano Lett. 2021, 21, 4500–4507.
Yun, T. Y.; Li, X. L.; Kim, S. H.; Moon, H. C. Dual-function electrochromic supercapacitors displaying real-time capacity in color. ACS Appl. Mater. Interfaces 2018, 10, 43993–43999.
Patel, K. J.; Bhatt, G. G.; Ray, J. R.; Suryavanshi, P.; Panchal, C. J. All-inorganic solid-state electrochromic devices: A review. J. Solid State Electrochem. 2017, 21, 337–347.
Gu, C.; Wang, S.; He, J. L.; Zhang, Y. M.; Zhang, S. X. A. High-durability organic electrochromic devices based on in-situ-photocurable electrochromic materials. Chem. 2023, 9, 2841–2854.
Tong, X. R.; Wang, J. H.; Zhang, P.; Lei, P. Y.; Gao, Y.; Ren, R. R.; Zhang, S. Y.; Zhu, R.; Cai, G. F. Insight into the structure-activity relationship in electrochromism of WO3 with rational internal cavities for broadband tunable smart windows. Chem. Eng. J. 2023, 470, 144130.
Wang, J. Y.; Zhao, W. X.; Tam, B.; Zhang, H. W.; Zhou, Y. R.; Yong, L.; Cheng, W. Pseudocapacitive porous amorphous vanadium pentoxide with enhanced multicolored electrochromism. Chem. Eng. J. 2023, 452, 139655.
Wen, R. T.; Granqvist, C. G.; Niklasson, G. A. Anodic electrochromism for energy-efficient windows: Cation/anion-based surface processes and effects of crystal facets in nickel oxide thin films. Adv. Funct. Mater. 2015, 25, 3359–3370.
Li, H. Z.; McRae, L.; Elezzabi, A. Y. Solution-processed interfacial PEDOT: PSS assembly into porous tungsten molybdenum oxide nanocomposite films for electrochromic applications. ACS Appl. Mater. Interfaces 2018, 10, 10520–10527.
Ma, Q.; Chen, J. X.; Zhang, H.; Su, Y. W.; Jiang, Y. J.; Dong, S. J. Dual-function self-powered electrochromic batteries with energy storage and display enabled by potential difference. ACS Energy Lett. 2023, 8, 306–313.
Qian, J. H.; Ma, D. Y.; Xu, Z. P.; Li, D.; Wang, J. M. Electrochromic properties of hydrothermally grown Prussian blue film and device. Sol. Energy Mater. Sol. Cells 2018, 177, 9–14.
Isfahani, V. B.; Memarian, N.; Dizaji, H. R.; Arab, A.; Silva, M. M. The physical and electrochromic properties of Prussian blue thin films electrodeposited on ITO electrodes. Electrochim. Acta 2019, 304, 282–291.
Hedley, L.; Porteous, L.; Hutson, D.; Robertson, N.; Johansson, J. O. Electrochromic bilayers of Prussian blue and its Cr analogue. J. Mater. Chem. C 2018, 6, 512–517.
Liao, H. Y.; Liao, T. C.; Chen, W. H.; Chang, C. H.; Chen, L. C. Molybdate hexacyanoferrate (MoOHCF) thin film: A brownish red Prussian blue analog for electrochromic window application. Sol. Energy Mater. Sol. Cells 2016, 145, 8–15.
Tajima, K.; Kubota, T.; Jeong, C. Y. Preparation of electrochromic thin films by humidity-controlled spin coating. Thin Solid Films 2022, 758, 139412.
Kim, S. Y.; Yun, T. Y.; Yu, K. S.; Moon, H. C. Reliable, high-performance electrochromic supercapacitors based on metal-doped nickel oxide. ACS Appl. Mater. Interfaces 2020, 12, 51978–51986.
Hasani, A.; Le, Q. V.; Nguyen, T. P.; Choi, K. S.; Sohn, W.; Jang, H. W.; Kim, S. Y. A thorough study on electrochromic properties of metal doped tungsten trioxide film prepared by a facile solution process. Electrochim. Acta 2018, 283, 1195–1202.
Hassan, M.; Abbas, G.; Lu, Y.; Wang, Z. Y.; Peng, Z. C. A smart flexible supercapacitor enabled by a transparent electrochromic electrode composed of W18O49 nanowires/rGO composite films. J. Mater. Chem. A 2022, 10, 4870–4880.
Neff, V. D. Electrochemical oxidation and reduction of thin films of Prussian blue. J. Electrochem. Soc. 1978, 125, 886–887.
Xu, H. B.; Gong, L. T.; Zhou, S. Y.; Cao, K. L.; Wang, S.; Zhao, J. P.; Li, Y. Enhancing the electrochromic stability of Prussian blue based on TiO2 nanorod arrays. New J. Chem. 2020, 44, 2236–2240.
Meng, Y.; Li, Z. X.; Liu, Z. F. Enhanced electrochromic properties of inorganic-organic tungsten oxide and Prussian blue core-shell film. J. Mater. Sci.: Mater. Electron. 2022, 33, 24995–25005.
Xu, L. Q.; Li, J. Y.; Li, L.; Luo, Z.; Xiang, Y. E.; Deng, W. N.; Zou, G. Q.; Hou, H. S.; Ji, X. B. Carbon dots evoked li ion dynamics for solid state battery. Small 2021, 17, 2102978.
Du, J. J.; Xu, N.; Fan, J. L.; Sun, W.; Peng, X. J. Carbon dots for in vivo bioimaging and theranostics. Small 2019, 15, 1805087.
Shejale, K. P.; Jaiswal, A.; Kumar, A.; Saxena, S.; Shukla, S. Nitrogen doped carbon quantum dots as Co-active materials for highly efficient dye sensitized solar cells. Carbon 2021, 183, 169–175.
Tang, Q. W.; Zhu, W. L.; He, B. L.; Yang, P. Z. Rapid conversion from carbohydrates to large-scale carbon quantum dots for all-weather solar cells. ACS Nano 2017, 11, 1540–1547.
Eivazzadeh-Keihan, R.; Bahojb Noruzi, E.; Chidar, E.; Jafari, M.; Davoodi, F.; Kashtiaray, A.; Ghafori Gorab, M.; Masoud Hashemi, S.; Javanshir, S.; Ahangari Cohan, R. et al. Applications of carbon-based conductive nanomaterials in biosensors. Chem. Eng. J. 2022, 442, 136183.
Duan, Q. Q.; Si, S.; Sang, S. B.; Wang, J. L.; Zhang, B. Y.; Guan, Z. W.; Jia, M. Y.; Xue, J. J. Study on the photothermal performance of supra-(carbon nanodots) developed with dicyandiamide N-doped. Colloids Surf. A Physicochem. Eng. Aspects 2022, 648, 129346.
Wang, Y. G.; Gong, Z. M.; Zeng, Y.; Zhao, H. L.; Yang, J. K. High-properties electrochromic device based on TiO2@graphene/Prussian blue core-shell nanostructures. Chem. Eng. J. 2022, 431, 134066.
Zhang, X.; Shen, Y. T.; Xu, S. P.; Yue, J.; Guo, Q.; Huang, D. S.; Yang, B.; Shi, W.; Liang, C. Y.; Xu, W. Q. Intracellular pH-propelled assembly of smart carbon nanodots and selective photothermal therapy for cancer cells. Colloids Surf. B Biointerf. 2020, 188, 110724.
Yin, Q.; Kelsall, G. H.; Vaughan, D. J.; England, K. E. R. Atmospheric and electrochemical oxidation of the surface of chalcopyrite (CuFeS2). Geochim. Cosmochim. Acta 1995, 59, 1091–1100.
Langevoort, J. C.; Sutherland, I.; Hanekamp, L. J.; Gellings, P. J. On the oxide formation on stainless steels AISI 304 and incoloy 800H investigated with XPS. Appl. Surf. Sci. 1987, 28, 167–179.
Srivastava, S.; Badrinarayanan, S.; Mukhedkar, A. J. X-ray photoelectron spectra of metal complexes of substituted 2,4-pentanediones. Polyhedron 1989, 4, 409–414.
Brant, P.; Feltham, R. D. X- ray photoelectron spectra of iron complexes: Correlation of iron 2p satellite intensity with complex spin state. J. Electron Spectrosc. Relat. Phenom. 1983, 32, 205–221.
Tang, J.; Xu, R. P.; Sui, G. X.; Guo, D. X.; Zhao, Z. L.; Fu, S. S.; Yang, X.; Li, Y.; Li, J. L. Double-shelled porous g-C3N4 nanotubes modified with amorphous Cu-doped FeOOH nanoclusters as 0D/3D non-homogeneous photo-Fenton catalysts for effective removal of organic dyes. Small 2023, 15, 2208232.
Mansour, A. N.; Ko, J. K.; Waller, G. H.; Martin, C. A.; Zhang, C.; Qiao, X. Y.; Wang, Y. C.; Zhou, X.; Balasubramanian, M. Structural analysis of K4Fe(CN)63H2O, K3Fe(CN)6 and Prussian blue. ECS J. Solid State Sci. Technol. 2021, 10, 103002.
Lee, T. H.; Rabalais, J. W. X- ray photoelectron spectra and electronic structure of some diamine compounds. J. Electron Spectrosc. Relat. Phenom. 1977, 11, 123–127.
Yatsimirskii, K. B.; Nemoshkalenko, V. V.; Nazarenko, Y. P.; Aleshin, V. G.; Zhilinskaya, V. V.; Tomashevsky, N. A. Use of X-ray photoelectron and Mö ssbauer spectroscopies in the study of iron pentacyanide complexes. J. Electron Spectrosc. Relat. Phenom. 1977, 10, 239–245.
Hu, C. W.; Sato, T.; Zhang, J.; Moriyama, S.; Higuchi, M. Three-dimensional Fe(II)-based metallo-supramolecular polymers with electrochromic properties of quick switching, large contrast, and high coloration efficiency. ACS Appl. Mater. Interfaces 2014, 6, 9118–9125.
Yan, S. M.; Fu, H. C.; Dong, Y. J.; Li, W. J.; Dai, Y. Y.; Zhang, C. Synthesis, electrochemistry and electrochromic properties of donor-acceptor conjugated polymers based on swivel-cruciform monomers with different central cores. Electrochim. Acta 2020, 354, 136672.
Dai, Y. Y.; Li, W. J.; Qu, X. X.; Liu, J.; Yan, S. M.; Ouyang, M.; Lv, X. J.; Zhang, C. Electrochemistry, electrochromic and color memory properties of polymer/copolymer based on novel dithienylpyrrole structure. Electrochim. Acta 2017, 229, 271–280.
Xu, M.; Wang, S.; Zhou, S. Y.; Sun, W. H.; Ding, Z. M.; Zhang, X.; Zhao, J. P.; Li, Y. Construction of TiO2@C@Prussian blue core-shell nanorod arrays for enhanced electrochromic switching speed and cycle stability. J. Alloys Compd. 2022, 908, 164410.
Xu, M.; Li, K.; Wang, S.; Zhou, S. Y.; Zhang, H. L.; Xu, H. B.; Zhao, J. P.; Li, Y. Designing TiO2/Au/Prussian blue heterostructures nanorod arrays for ultra-stable cycle and ultra-fast response electrochromism. Nano Res. 2023, 16, 3294–3303.
Acknowledgements
This work was supported by Jiangsu Specially Appointed Professor program, the Tsinghua-Toyota Joint Research Fund, and the National Key Research and Development Program of China (Nos. 2020YFC2201103 and 2020YFA0210702).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2024_6415_MOESM1_ESM.pdf
Nitrogen-doped carbon dots-modified Prussian blue with sandwich-like structure as a high-performance electrochromic material
Rights and permissions
About this article
Cite this article
Zhang, Y., Li, L., Zhao, S. et al. Nitrogen-doped carbon dots-modified Prussian blue with sandwich-like structure as a high-performance electrochromic material. Nano Res. (2024). https://doi.org/10.1007/s12274-024-6415-x
Received:
Revised:
Accepted:
Published:
DOI: https://doi.org/10.1007/s12274-024-6415-x