Abstract
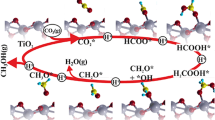
The selective oxidation of methane under mild conditions remains the “Holy Grail of Catalysis”. The key to activating methane and inhibiting over-oxidation of target oxygenates lies in designing active centers. Copper nanoparticles were loaded onto TiO2 nanofibers using the photo-deposition method. The resulting catalysts were found to effectively convert methane into C1 oxygenated products under mild conditions. Compared with previously reported catalysts, it delivers a superior performance of up to 2510.7 mmol·gCu−1·hr−1 productivity with a selectivity of around 100% at 80 °C for 5 min. Microstructure characterizations and density functional theory (DFT) calculations indicate that TiO2 in the mixed phase of anatase and rutile significantly increases the Cu+/Cu0 ratio of the supported Cu species, and this ratio is linearly related to the formation rate of oxygen-containing species. The Cu1 site promotes the generation of active O species from H2O2 dissociation on Cu2O (111). These active O species reduce the energy barrier for breaking the C–H bond of CH4, thus boosting the catalytic activity. The methane conversion mechanism was proposed as a methyl radical pathway to form CH3OH and CH3OOH, and then the generated CH3OH is further oxidized to HOCH2OOH.

Similar content being viewed by others
References
Sun, X.; Chen, X. Y.; Fu, C.; Yu, Q. B.; Zheng, X. S.; Fang, F.; Liu, Y. X.; Zhu, J. F.; Zhang, W. H.; Huang, W. X. Molecular oxygen enhances H2O2 utilization for the photocatalytic conversion of methane to liquid-phase oxygenates. Nat. Commun. 2022, 13, 6677.
Chen, F. Q.; Zheng, W.; Zhu, N.; Cheng, D. G.; Zhan, X. L. Oxidative coupling of methane over Na-W-Mn-Zr-S-P/SiO2 catalyst: Effect of S, P addition on the catalytic performance. Catal. Lett. 2008, 125, 348–351.
Lunsford, J. H. The catalytic oxidative coupling of methane. Angew. Chem. Int. Ed. Engl. 1995, 34, 970–980.
Kwapien, K.; Paier, J.; Sauer, J.; Geske, M.; Zavyalova, U.; Horn, R.; Schwach, P.; Trunschke, A.; Schlögl, R. Sites for methane activation on lithium-doped magnesium oxide surfaces. Angew. Chem., Int. Ed. 2014, 53, 8774–8778.
Xu, Y. D.; Bao, X. H.; Lin, L. W. Direct conversion of methane under nonoxidative conditions. J. Catal. 2003, 216, 386–395.
Hutchings, G. J.; Scurrell, M. S.; Woodhouse, J. R. Oxidative coupling of methane using oxide catalysts. Chem. Soc. Rev. 1989, 18, 251–283.
Guo, X. G.; Fang, G. Z.; Li, G.; Ma, H.; Fan, H. J.; Yu, L.; Ma, C.; Wu, X.; Deng, D. H.; Wei, M. M. et al. Direct, nonoxidative conversion of methane to ethylene, aromatics, and hydrogen. Science 2014, 344, 616–619.
Yu, X.; Zholobenko, V. L.; Moldovan, S.; Hu, D.; Wu, D.; Ordomsky, V. V.; Khodakov, A. Y. Stoichiometric methane conversion to ethane using photochemical looping at ambient temperature. Nat. Energy 2020, 5, 511–519.
Schulz, H. Short history and present trends of Fischer-Tropsch synthesis. Appl. Catal. A: Gen. 1999, 186, 3–12.
Sushkevich, V. L.; Palagin, D.; Ranocchiari, M.; van Bokhoven, J. A. Selective anaerobic oxidation of methane enables direct synthesis of methanol. Science 2017, 356, 523–527.
Chen, X. W.; Peng, M.; Cai, X. B.; Chen, Y. L.; Jia, Z. M.; Deng, Y. C.; Mei, B. B.; Jiang, Z.; Xiao, D. Q.; Wen, X. D. et al. Regulating coordination number in atomically dispersed Pt species on defect-rich graphene for n-butane dehydrogenation reaction. Nat. Commun. 2021, 12, 2664.
Chen, X. W.; Qin, X. T.; Jiao, Y. Y.; Peng, M.; Diao, J. Y.; Ren, P. J.; Li, C. Y.; Xiao, D. Q.; Wen, X. D.; Jiang, Z. et al. Structure-dependence and metal-dependence on atomically dispersed Ir catalysts for efficient n-butane dehydrogenation. Nat. Commun. 2023, 14, 2588.
Ab Rahim, M. H.; Forde, M. M.; Jenkins, R. L.; Hammond, C.; He, Q.; Dimitratos, N.; Lopez-Sanchez, J. A.; Carley, A. F.; Taylor, S. H.; Willock, D. J. et al. Oxidation of methane to methanol with hydrogen peroxide using supported gold-palladium alloy nanoparticles. Angew. Chem., Int. Ed. 2013, 52, 1280–1284.
Hammond, C.; Forde, M. M.; Ab Rahim, M. H.; Thetford, A.; He, Q.; Jenkins, R. L.; Dimitratos, N.; Lopez-Sanchez, J. A.; Dummer, N. F.; Murphy, D. M. et al. Direct catalytic conversion of methane to methanol in an aqueous medium by using copper-promoted Fe-ZSM-5. Angew. Chem., Int. Ed. 2012, 51, 5129–5133.
Armstrong, R. D.; Peneau, V.; Ritterskamp, N.; Kiely, C. J.; Taylor, S. H.; Hutchings, G. J. The role of copper speciation in the low temperature oxidative upgrading of short chain alkanes over Cu/ZSM-5 catalysts. ChemPhysChem 2018, 19, 469–478.
Zhao, W. S.; Shi, Y. N.; Jiang, Y. H.; Zhang, X. F.; Long, C.; An, P. F.; Zhu, Y. F.; Shao, S. X.; Yan, Z.; Li, G. D. et al. Fe-O clusters anchored on nodes of metal-organic frameworks for direct methane oxidation. Angew. Chem., Int. Ed. 2021, 60, 5811–5815.
Yang, L.; Huang, J. X.; Ma, R.; You, R.; Zeng, H.; Rui, Z. B. Metal-organic framework-derived IrO2/CuO catalyst for selective oxidation of methane to methanol. ACS Energy Lett. 2019, 4, 2945–2951.
Shen, Q. K.; Cao, C. Y.; Huang, R. K.; Zhu, L.; Zhou, X.; Zhang, Q. H.; Gu, L.; Song, W. G. Single chromium atoms supported on titanium dioxide nanoparticles for synergic catalytic methane conversion under mild condition. Angew. Chem., Int. Ed. 2020, 59, 1216–1219.
Agarwal, N.; Freakley, S. J.; McVicker, R. U.; Althahban, S. M.; Dimitratos, N.; He, Q.; Morgan, D. J.; Jenkins, R. L.; Willock, D. J.; Taylor, S. H. et al. Aqueous Au-Pd colloids catalyze selective CH4 oxidation to CH3OH with O2 under mild conditions. Science 2017, 358, 223–227.
Mahlaba, S. V. L.; Hytoolakhan Lal Mahomed, N.; Govender, A.; Guo, J. F.; Leteba, G. M.; Cilliers, P. L.; van Steen, E. Platinum-catalysed selective aerobic oxidation of methane to formaldehyde in the presence of liquid water. Angew. Chem., Int. Ed. 2022, 61, e202206841.
Zhang, P. Y.; Liu, H. Y.; Li, X. M. Photo-reduction synthesis of Cu nanoparticles as Plasmon-driven non-semiconductor photocatalyst for overall water splitting. Appl. Surf. Sci. 2021, 535, 147720.
Wu, X. Y.; Zeng, Y.; Liu, H. C.; Zhao, J. Q.; Zhang, T. R.; Wang, S. L. Noble-metal-free dye-sensitized selective oxidation of methane to methanol with green light (550 nm). Nano Res. 2021, 14, 4584–4590.
Guo, X. Y.; Hu, Z.; Lv, J. X.; Li, H.; Zhang, Q. H.; Gu, L.; Zhou, W.; Zhang, J. W.; Hu, S. Fine- tuning of Pd-Rh core–shell catalysts by interstitial hydrogen doping for enhanced methanol oxidation. Nano Res. 2022, 15, 1288–1294.
Guo, H. M.; Wu, L.; Nie, S. Y.; Yang, D. R.; Wang, X. Ultrathin zirconium-porphyrin based nanobelts as photo-coupled electrocatalysis for CH4 oxidation to CO. Nano Res. 2023, 16, 12641–12646.
Zeng, F.; Zhang, J.; Xu, R.; Zhang, R. J.; Ge, J. P. Highly dispersed Ni/MgO-mSiO2 catalysts with excellent activity and stability for dry reforming of methane. Nano Res. 2022, 15, 5004–5013.
Nie, J.; Patrocinio, A. O. T.; Hamid, S.; Sieland, F.; Sann, J.; Xia, S.; Bahnemann, D. W.; Schneider, J. New insights into the plasmonic enhancement for photocatalytic H2 production by Cu-TiO2 upon visible light illumination. Phys. Chem. Chem. Phys. 2018, 20, 5264–5273.
DeSario, P. A.; Pietron, J. J.; Brintlinger, T. H.; McEntee, M.; Parker, J. F.; Baturina, O.; Stroud, R. M.; Rolison, D. R. Oxidation-stable plasmonic copper nanoparticles in photocatalytic TiO2 nanoarchitectures. Nanoscale 2017, 9, 11720–11729.
Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50.
Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775.
Perdew, J. P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868.
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 1994, 50, 17953–17979.
Grimme, S.; Antony, J.; Ehrlich, S.; Krieg, H. A consistent and accurate ab initio parametrization of density functional dispersion correction (DFT-D) for the 94 elements H-Pu. J. Chem. Phys. 2010, 132, 154104.
Grimme, S. Semiempirical GGA-type density functional constructed with a long-range dispersion correction. J. Comput. Chem. 2006, 27, 1787–1799.
Monkhorst, H. J.; Pack, J. D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192.
Qiu, X. Q.; Miyauchi, M.; Sunada, K.; Minoshima, M.; Liu, M.; Lu, Y.; Li, D.; Shimodaira, Y.; Hosogi, Y.; Kuroda, Y. et al. Hybrid CuxO/TiO2 nanocomposites as risk-reduction materials in indoor environments. ACS Nano 2012, 6, 1609–1618.
Zhang, Q. Y.; Li, Y.; Ackerman, E. A.; Gajdardziska-Josifovska, M.; Li, H. L. Visible light responsive iodine-doped TiO2 for photocatalytic reduction of CO2 to fuels. Appl. Catal. A: Gen. 2011, 400, 195–202.
Deiana, C.; Fois, E.; Coluccia, S.; Martra, G. Surface structure of TiO2 P25 nanoparticles: Infrared study of hydroxy groups on coordinative defect sites. J. Phys. Chem. C 2010, 114, 21531–21538.
Zhang, J.; Li, M. J.; Feng, Z. C.; Chen, J.; Li, C. UV Raman spectroscopic study on TiO2. I. phase transformation at the surface and in the bulk. J. Phys. Chem. B 2006, 110, 927–935.
Espinós, J. P.; Morales, J.; Barranco, A.; Caballero, A.; Holgado, J. P.; González-Elipe, A. R. Interface effects for Cu, CuO, and Cu2O Deposited on SiO2 and ZrO2. XPS determination of the valence state of copper in Cu/SiO2 and Cu/ZrO2 catalysts. J. Phys. Chem. B 2002, 106, 6921–6929.
Dan, Z. H.; Yang, Y. L.; Qin, F. X.; Wang, H.; Chang, H. Facile fabrication of Cu2O nanobelts in ethanol on nanoporous Cu and their photodegradation of methyl orange. Materials 2018, 11, 446.
Zhen, W. L.; Jiao, W. J.; Wu, Y. Q.; Jing, H. W.; Lu, G. X. The role of a metallic copper interlayer during visible photocatalytic hydrogen generation over a Cu/Cu2O/Cu/TiO2 catalyst. Catal. Sci. Technol. 2017, 7, 5028–5037.
Wang, Y. T.; Zhou, W.; Jia, R. R.; Yu, Y. F.; Zhang, B. Unveiling the activity origin of a copper-based electrocatalyst for selective nitrate reduction to ammonia. Angew. Chem., Int. Ed. 2020, 59, 5350–5354.
Zhou, J. J.; Pan, F.; Yao, Q. F.; Zhu, Y. Q.; Ma, H. R.; Niu, J. F.; Xie, J. P. Achieving efficient and stable electrochemical nitrate removal by in-situ reconstruction of Cu2O/Cu electroactive nanocatalysts on Cu foam. Appl. Catal. B: Environ. 2022, 317, 121811.
Jin, Z.; Wang, L.; Zuidema, E.; Mondal, K.; Zhang, M.; Zhang, J.; Wang, C. T.; Meng, X. J.; Yang, H. Q.; Mesters, C. et al. Hydrophobic zeolite modification for in situ peroxide formation in methane oxidation to methanol. Science 2020, 367, 193–197.
Fang, G. Q.; Hu, J. N.; Tian, L. C.; Liang, J. X.; Lin, J.; Li, L.; Zhu, C.; Wang, X. D. Zirconium-oxo nodes of MOFs with tunable electronic properties provide effective OH species for enhanced methane hydroxylation. Angew. Chem., Int. Ed. 2022, 61, e202205077.
Fan, J. C.; Liang, S. X.; Zhu, K. X.; Mao, J.; Cui, X. J.; Ma, C.; Yu, L.; Deng, D. H. Boosting room-temperature conversion of methane via confining Cu atoms in ultrathin Ru nanosheets. Chem Catal. 2022, 2, 2253–2261.
Li, W. C.; Li, Z.; Zhang, H.; Liu, P. X.; Xie, Z. A.; Song, W. Y.; Liu, B. J.; Zhao, Z. Efficient catalysts of surface hydrophobic Cu-BTC with coordinatively unsaturated Cu(I) sites for the direct oxidation of methane. Proc. Natl. Acad. Sci. USA 2023, 120, e2206619120.
Wu, L. N.; Tian, Z. Y.; Qin, W. DFT study on CO catalytic oxidation mechanism on the defective Cu2O (111) surface. J. Phys. Chem. C 2018, 122, 16733–16740.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Nos. 92145301, 91845201, 22002094, 22102106, and 22309061) and the Natural Science Foundation of Jilin Province (No. YDZJ202201ZYTS360).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2023_6356_MOESM1_ESM.pdf
TiO2 nanofiber-supported copper nanoparticle catalysts for highly efficient methane conversion to C1 oxygenates under mild conditions
Rights and permissions
About this article
Cite this article
Li, W., Ren, Y., Xie, Z. et al. TiO2 nanofiber-supported copper nanoparticle catalysts for highly efficient methane conversion to C1 oxygenates under mild conditions. Nano Res. 17, 3844–3852 (2024). https://doi.org/10.1007/s12274-023-6356-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6356-9