Abstract
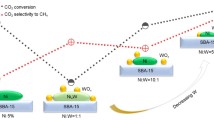
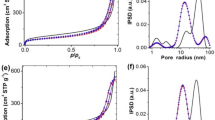
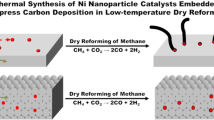
Highly dispersed Ni catalyst and alkaline promoters supported by mesoporous SiO2 nanospheres were synthesized and applied as an active and stable catalyst for dry reforming of methane (DRM). The as-prepared Ni/MgO-mSiO2 catalyst showed stable conversions of CH4 and CO2 around 82% and 85% in 120 h of DRM reaction, which was superior in performance compared to similar catalysts in literatures. Based on the transmission electron microscope (TEM) images, energy-dispersive spectroscopy (EDS), CO-pulse adsorption, temperature programmed reduction of the oxidized catalysts by hydrogen (H2-TPR), X-ray photoelectron spectroscopy (XPS), temperature-programmed desorption of CO2 (CO2-TPD), and thermal gravitational analysis (TGA), the promotion effect of MgO on the Ni catalyst was systematically studied. The introduction of Mg2+ in synthesis enhanced the interaction between Ni2+ and mSiO2, which led to a high dispersion of active centers and a strong “metal-support” interactions to inhibit the sintering and deactivation of Ni at reaction temperatures. On the other hand, Ni and MgO nanoparticles formed adjacently on mSiO2, where the “Ni-MgO” interface not only improved the Ni0 distribution and promoted the cracking of CH4 but also promoted the activation of CO2 and the elimination of carbon deposits. A high and stable conversion of CH4 and CO2 were then achieved through the synergistic effect of Ni catalyst, MgO promoter, and mSiO2 support.

Similar content being viewed by others
References
He, M. Y.; Sun, Y. H.; Han, B. X. Green carbon science: Scientific basis for integrating carbon resource processing, utilization, and recycling. Angew. Chem., Int. Ed. 2013, 52, 9620–9633.
Melikoglu, M. Shale gas: Analysis of its role in the global energy market. Renew. Sust. Energy Rev. 2014, 37, 460–468.
Olah, G. A.; Surya Prakash, G. K.; Goeppert, A. Anthropogenic chemical carbon cycle for a sustainable future. J. Am. Chem. Soc. 2011, 133, 12881–12898.
Olah, G. A. Towards oil independence through renewable methanol chemistry. Angew. Chem., Int. Ed. 2013, 52, 104–107.
Chen, W. Y.; Liu, X. M.; Han, B.; Liang, S. J.; Deng, H.; Lin, Z. Boosted photoreduction of diluted CO2 through oxygen vacancy engineering in NiO nanoplatelets. Nano Res. 2021, 14, 730–737.
Li, Z. H.; Shi, R.; Zhao, J. Q.; Zhang, T. R. Ni-based catalysts derived from layered-double-hydroxide nanosheets for efficient photothermal CO2 reduction under flow-type system. Nano Res. 2021, 14, 4828–4832.
Wu, X. Y.; Zeng, Y.; Liu, H. C.; Zhao, J. Q.; Zhang, T. R.; Wang, S. L. Noble-metal-free dye-sensitized selective oxidation of methane to methanol with green light (550 nm). Nano Res. 2021, 14, 4584–4590.
Xie, H. P.; Lan, C.; Chen, B.; Wang, F. H.; Liu, T. Noble-metal-free catalyst with enhanced hydrogen evolution reaction activity based on granulated Co-doped Ni-Mo phosphide nanorod arrays. Nano Res. 2020, 13, 3321–3329.
Zhang, M.; Hu, Z.; Gu, L.; Zhang, Q. H.; Zhang, L. H.; Song, Q.; Zhou, W.; Hu, S. Electrochemical conversion of CO2 to syngas with a wide range of CO/H2 ratio over Ni/Fe binary single-atom catalysts. Nano Res. 2020, 13, 3206–3211.
Bhavani, A. G.; Kim, W. Y.; Lee, J. S. Barium substituted lanthanum manganite perovskite for CO2 reforming of methane. ACS Catal. 2013, 3, 1537–1544.
Beheshti Askari, A.; Al Samarai, M.; Morana, B.; Tillmann, L.; Pfänder, N.; Wandzilak, A.; Watts, B.; Belkhou, R.; Muhler, M.; DeBeer, S. In situ X-ray microscopy reveals particle dynamics in a NiCo dry methane reforming catalyst under operating conditions. ACS Catal. 2020, 10, 6223–6230.
Wang, W.; Wang, S. P.; Ma, X. B.; Gong, J. H. Recent advances in catalytichydrogenation of carbon dioxide. Chem. Soc. Rev. 2011, 40, 3703–3727.
Pakhare, D.; Spivey, J. A review of dry (CO2) reforming of methane over noble metal catalysts. Chem. Soc. Rev. 2014, 43, 7813–7837.
Sheng, K. F.; Luan, D.; Jiang, H.; Zeng, F.; Wei, B.; Pang, F.; Ge, J. P. NixCoy nanocatalyst supported by ZrO2 hollow sphere for dry reforming of methane: Synergetic catalysis by Ni and Co in alloy. ACS Appl. Mater. Interfaces 2019, 11, 24078–24087.
Tian, J. Q.; Ma, B.; Bu, S. Y.; Yuan, Q. H.; Zhao, C. One-pot synthesis of highly sintering- and coking-resistant Ni nanoparticles encapsulated in dendritic mesoporous SiO2 for methane dry reforming. Chem. Commun. 2018, 54, 13993–13996.
Horváth, Á.; Baán, K.; Varga, E.; Oszkó, A.; Vágó, Á.; Törő, M.; Erdőhelyi, A. Dry reforming of CH4 on Co/Al2O3 catalysts reduced at different temperatures. Catal. Today 2017, 281, 233–240.
Ewbank, J. L.; Kovarik, L.; Kenvin, C. C.; Sievers, C. Effect of preparation methods on the performance of Co/Al2O3 catalysts for dry reforming of methane. Green Chem. 2014, 16, 885–896.
Soykal, I. I.; Sohn, H.; Ozkan, U. S. Effect of support particle size in steam reforming of ethanol over Co/CeO2 catalysts. ACS Catal. 2012, 2, 2335–2348.
Kim, S. M.; Abdala, P. M.; Margossian, T.; Hosseini, D.; Foppa, L.; Armutlulu, A.; van Beek, W.; Comas-Vives, A.; Copéret, C.; Müller, C. Cooperativity and dynamics increase the performance of NiFe dry reforming catalysts. J. Am. Chem. Soc. 2017, 139, 1937–1949.
Theofanidis, S. A.; Galvita, V. V.; Poelman, H.; Marin, G. B. Enhanced carbon-resistant dry reforming Fe-Ni catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039.
Li, S. R.; Gong, J. L. Strategies for improving the performance and stability of Ni-based catalysts for reforming reactions. Chem. Soc. Rev. 2014, 43, 7245–7256.
Wang, Y.; Yao, L.; Wang, S. H.; Mao, D. H.; Hu, C. W. Low-temperature catalytic CO2 dry reforming of methane on Ni-based catalysts: A review. Fuel Process. Technol. 2018, 169, 199–206.
Usman, M.; Wan Daud, W. M. A.; Abbas, H. F. Dry reforming of methane: Influence of process parameters—A review. Renew. Sust. Energy Rev. 2015, 45, 710–744.
Abdulrasheed, A.; Jalil, A. A.; Gambo, Y.; Ibrahim, M.; Hambali, H. U.; Shahul Hamid, M. Y. A review on catalyst development for dry reforming of methane to syngas: Recent advances. Renew. Sust. Energy Rev. 2019, 108, 175–193.
Singh, S.; Zubenko, D.; Rosen, B. A. Influence of LaNiO3 shape on its solid-phase crystallization into coke-free reforming catalysts. ACS Catal. 2016, 6, 4199–4205.
Nair, M. M.; Kaliaguine, S.; Kleitz, F. Nanocast LaNiO3 perovskites as precursors for the preparation of coke-resistant dry reforming catalysts. ACS Catal. 2014, 4, 3837–3846.
Aramouni, N. A. K.; Touma, J. G.; Tarboush, B. A.; Zeaiter, J.; Ahmad, M. N. Catalyst design for dry reforming of methane: Analysis review. Renew. Sust. Energy Rev. 2018, 82, 2570–2585.
Abou Rached, J. A.; Cesario, M. R.; Estephane, J.; Tidahy, H. L.; Gennequin, C.; Aouad, S.; Aboukaïs, A.; Abi-Aad, E. Effects of cerium and lanthanum on Ni-based catalysts for CO2 reforming of toluene. J. Environ. Chem. Eng. 2018, 6, 4743–4754.
Zhang, L.; Wang, X. G.; Chen, C. J.; Zou, X. J.; Shang, X. F.; Ding, W. Z.; Lu, X. G. Investigation of mesoporous NiAl2O4/MOx (M = La, Ce, Ca, Mg)-γ-Al2O3 nanocomposites for dry reforming of methane. RSC Adv. 2017, 7, 33143–33154.
Liu, H. R.; Wierzbicki, D.; Debek, R.; Motak, M.; Grzybek, T.; Da Costa, P.; Gálvez, M. E. La-promoted Ni-hydrotalcite-derived catalysts for dry reforming of methane at low temperatures. Fuel 2016, 182, 8–16.
Xu, L. L.; Song, H. L.; Chou, L. J. Carbon dioxide reforming of methane over ordered mesoporous NiO-MgO-Al2O3 composite oxides. Appl. Catal. B 2011, 108–109, 177–190.
Zhao, Y. C.; Liu, B. S.; Amin, R. CO2 reforming of CH4 over MgO-doped Ni/MAS-24 with microporous ZSM-5 structure. Ind. Eng. Chem. Res. 2016, 55, 6931–6942.
Zeng, Y. X.; Wang, L.; Wu, C. F.; Wang, J. Q.; Shen, B. X.; Tu, X. Low temperature reforming of biogas over K-, Mg- and Ce- promoted Ni/Al2O3 catalysts for the production of hydrogen rich syngas: Understanding the plasma-catalytic synergy. Appl. Catal. B 2018, 224, 469–478.
Feng, X. Q.; Liu, J.; Zhang, P.; Zhang, Q.; Xu, L. Y.; Zhao, L. P.; Song, X. F.; Gao, L. Highly coke resistant Mg-Ni/Al2O3 catalyst prepared via a novel magnesiothermic reduction for methane reforming catalysis with CO2: The unique role of Al-Ni intermetallics. Nanoscale 2019, 11, 1262–1272.
Taherian, Z.; Yousefpour, M.; Tajally, M.; Khoshandam, B. A comparative study of ZrO2, Y2O3 and Sm2O3 promoted Ni/SBA-15 catalysts for evaluation of CO2/methane reforming performance. Int. J. Hydrogen Energy 2017, 42, 16408–16420.
Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni-Co catalysts and its effect on dry reforming of CH4. J. CO2Util. 2015, 10, 67–77.
Mo, W. L.; Ma, F. Y.; Ma, Y. Y.; Fan, X. The optimization of Ni-Al2O3 catalyst with the addition of La2O3 for CO2-CH4 reforming to produce syngas. Int. J. Hydrogen Energy 2019, 44, 24510–24524.
Múnera, J.; Faroldi, B.; Frutis, E.; Lombardo, E.; Cornaglia, L.; Carrazán, S. G. Supported Rh nanoparticles on CaO-SiO2 binary systems for the reforming of methane by carbon dioxide in membrane reactors. Appl. Catal. A 2014, 474, 114–124.
Singha, R. K.; Yadav, A.; Shukla, A.; Iqbal, Z.; Pendem, C.; Sivakumar, K.; Bal, R. Promoting effect of CeO2 and MgO for CO2 reforming of methane over Ni-ZnO catalyst. ChemistrySelect 2016, 1, 3075–3085.
Yan, X. L.; Hu, T.; Liu, P.; Li, S.; Zhao, B. R.; Zhang, Q.; Jiao, W. Y.; Chen, S.; Wang, P. F.; Lu, J. J. et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO2. Appl. Catal. B 2019, 246, 221–231.
Rezaei, M.; Alavi, S. M. Dry reforming over mesoporous nanocrystalline 5% Ni/M-MgAl2O4 (M: CeO2, ZrO2, La2O3) catalysts. Int. J. Hydrogen Energy 2019, 44, 16516–16525.
Khajenoori, M.; Rezaei, M.; Meshkani, F. Dry reforming over CeO2-promoted Ni/MgO nano-catalyst: Effect of Ni loading and CH4/CO2 molar ratio. J. Ind. Eng. Chem. 2015, 21, 717–722.
Abdulrasheed, A. A.; Jalil, A. A.; Hamid, M. Y. S.; Siang, T. J.; Abdullah, T. A. T. Dry reforming of CH4 over stabilized Ni-La@KCC-1 catalyst: Effects of La promoter and optimization studies using RSM. J. CO2Util. 2020, 37, 230–239.
Lu, Y.; Guo, D.; Zhao, Y. F.; Moyo, P. S.; Zhao, Y. J.; Wang, S. P.; Ma, X. B. Enhanced catalytic performance of Nix-V@HSS catalysts for the DRM reaction: The study of interfacial effects on Ni-VOx structure with a unique yolk-shell structure. J. Catal. 2021, 396, 65–80.
Polshettiwar, V.; Cha, D.; Zhang, X. X.; Basset, J. M. High-surfacearea silica nanospheres (KCC-1) with a fibrous morphology. Angew. Chem., Int. Ed. 2010, 49, 9652–9656.
Peng, H. G.; Zhang, X. H.; Han, X.; You, X. J.; Lin, S. X.; Chen, H.; Liu, W. M.; Wang, X.; Zhang, N.; Wang, Z. et al. Catalysts in coronas: A surface spatial confinement strategy for high-performance catalysts in methane dry reforming. ACS Catal. 2019, 9, 9072–9080.
Alipour, Z.; Rezaei, M.; Meshkani, F. Effect of alkaline earth promoters (MgO, CaO, and BaO) on the activity and coke formation of Ni catalysts supported on nanocrystalline Al2O3 in dry reforming of methane. J. Ind. Eng. Chem. 2014, 20, 2858–2863.
Wu, P.; Tao, Y. W.; Ling, H. J.; Chen, Z. B.; Ding, J.; Zeng, X.; Liao, X. Z.; Stampfl, C.; Huang, J. Cooperation of Ni and CaO at interface for CO2 reforming of CH4: A combined theoretical and experimental study. ACS Catal. 2019, 9, 10060–10069.
Liu, Q.; Zhong, Z. Y.; Gu, F. N.; Wang, X. Y.; Lu, X. P.; Li, H. F.; Xu, G. W.; Su, F. B. CO methanation on ordered mesoporous Ni-Cr-Al catalysts: Effects of the catalyst structure and Cr promoter on the catalytic properties. J. Catal. 2016, 337, 221–232.
Dou, J.; Zhang, R. G.; Hao, X. B.; Bao, Z. H.; Wu, T. P.; Wang, B. J.; Yu, F. Sandwiched SiO2@Ni@ZrO2 as a coke resistant nanocatalyst for dry reforming of methane. Appl. Catal. B 2019, 254, 612–623.
Baudouin, D.; Margossian, T.; Rodemerck, U.; Webb, P. B.; Veyre, L.; Krumeich, F.; Candy, J. P.; Thieuleux, C.; Copéret, C. Origin of the improved performance in lanthanum-doped silica-supported Ni catalysts. ChemCatChem 2017, 9, 586–596.
Bian, Z. F.; Kawi, S. Sandwich-like silica@Ni@silica multicore—shell catalyst for the low-temperature dry reforming of methane: Confinement effect against carbon formation. ChemCatChem 2018, 10, 320–328.
Lovell, E. C.; Fuller, A.; Scott, J.; Amal, R. Enhancing Ni-SiO2 catalysts for the carbon dioxide reforming of methane: Reduction-oxidation-reduction pre-treatment. Appl. Catal. B 2016, 199, 155–165.
Xu, L. L.; Song, H. L.; Chou, L. J. One-pot synthesis of ordered mesoporous NiO-CaO-Al2O3 composite oxides for catalyzing CO2 reforming of CH4. ACS Catal. 2012, 2, 1331–1342.
Kalai, D. Y.; Stangeland, K.; Jin, Y. Y.; Tucho, W. M.; Yu, Z. X. Biogas dry reforming for syngas production on La promoted hydrotalcite-derived Ni catalysts. Int. J. Hydrogen Energy 2018, 43, 19438–19450.
Acknowledgements
This work was supported by SINOPEC Research Institute of Petroleum Processing, the National Key Research and Development Program of China (No. 2016YFB0701103) and the National Natural Science Foundation of China (Nos. 21972046 and 22172054).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Zeng, F., Zhang, J., Xu, R. et al. Highly dispersed Ni/MgO-mSiO2 catalysts with excellent activity and stability for dry reforming of methane. Nano Res. 15, 5004–5013 (2022). https://doi.org/10.1007/s12274-022-4180-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4180-2