Abstract
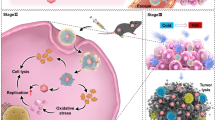
Bacterial outer membrane vesicles (OMVs) are potent immunostimulants of regulating the tumor microenvironment (TME) for immunotherapy, and can be used to deliver drugs. However, the severe systemic inflammatory response triggered by OMVs upon intravenous (i.v.) injection has limited their application. Here, we developed a safe and effective strategy by conjugating doxorubicin-loaded serum albumin (SA-DOX, AD) onto the surface of OMVs using a matrix metalloproteinase (MMP)-cleavable peptide linker (cL). This approach enabled the dynamic shielding of OMVs to reduce the systemic side effects while simultaneously enhancing the anti-tumor effects through chemo-immunotherapy. Specifically, the resulting OMV-cL-AD formulation exhibited significantly enhanced accumulation at the tumor site after i.v. administration, facilitated by the SA decoration on the OMVs surface. Subsequently, the shield on the OMV-cL-AD was cleaved by the over-expressed MMP in the TME, leading to the release of both OMVs and AD. This process provided OMV-induced immunotherapy and DOX-induced chemotherapy, resulting in synergistic tumor inhibition. In conclusion, our work demonstrated the potential of OMV-cL-AD as an effective immunochemotherapy strategy that can prolong the survival time of mice without inducing side effects.

Similar content being viewed by others
References
Dai, H. X.; Fan, Q.; Wang, C. Recent applications of immunomodulatory biomaterials for disease immunotherapy. Exploration 2022, 2, 20210157.
Long, Q.; Zheng, P.; Zheng, X.; Li, W. R.; Hua, L. Q.; Yang, Z. Q.; Huang, W. W.; Ma, Y. B. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv. Drug Deliv. Rev. 2022, 186, 114321.
Suri, K.; D’Souza, A.; Huang, D.; Bhavsar, A.; Amiji, M. Bacterial extracellular vesicle applications in cancer immunotherapy. Bioact. Mater. 2023, 22, 551–566.
Cao, Z. P.; Liu, J. Y. Bacteria and bacterial derivatives as drug carriers for cancer therapy. J. Control. Release 2020, 326, 396–407.
Kang, S. R.; Nguyen, D. H.; Yoo, S. W.; Min, J. J. Bacteria and bacterial derivatives as delivery carriers for immunotherapy. Adv. Drug Deliv. Rev. 2022, 181, 114085.
Zhuang, W. R.; Wang, Y. F.; Lei, Y.; Zuo, L. P.; Jiang, A. Q.; Wu, G. H.; Nie, W. D.; Huang, L. L.; Xie, H. Y. Phytochemical engineered bacterial outer membrane vesicles for photodynamic effects promoted immunotherapy. Nano Lett. 2022, 22, 4491–4500.
Li, Y.; Zhang, K. Y.; Wu, Y.; Yue, Y. L.; Cheng, K. M.; Feng, Q. Q.; Ma, X. T.; Liang, J.; Ma, N. N.; Liu, G. N. et al. Antigen capture and immune modulation by bacterial outer membrane vesicles as in situ vaccine for cancer immunotherapy post-photothermal therapy. Small 2022, 18, e2107461.
Chen, Q.; Bai, H. Z.; Wu, W. T.; Huang, G. J.; Li, Y.; Wu, M.; Tang, G. P.; Ping, Y. Bioengineering bacterial vesicle-coated polymeric nanomedicine for enhanced cancer immunotherapy and metastasis prevention. Nano Lett. 2020, 20, 11–21.
Schwechheimer, C.; Kuehn, M. J. Outer-membrane vesicles from gram-negative bacteria: Biogenesis and functions. Nat. Rev. Microbiol. 2015, 13, 605–619.
Chen, S. L.; Lei, Q.; Zou, X. H.; Ma, D. D. The role and mechanisms of gram-negative bacterial outer membrane vesicles in inflammatory diseases. Front. Immunol. 2023, 14, 1157813.
Kulp, A.; Kuehn, M. J. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu. Rev. Microbiol. 2010, 64, 163–184.
Cao, Z. P.; Liu, R.; Wang, C. H.; Lin, S. S.; Wang, L.; Pang, Y. Fluorescence-activating and absorption-shifting nanoprobes for anaerobic tracking of gut microbiota derived vesicles. ACS Nano 2023, 17, 2279–2293.
Wang, X. Y.; Lin, S. S.; Wang, L.; Cao, Z. P.; Zhang, M. M.; Zhang, Y. F.; Liu, R.; Liu, J. Y. Versatility of bacterial outer membrane vesicles in regulating intestinal homeostasis. Sci. Adv. 2023, 9, eade5079.
Qing, S.; Lyu, C.; Zhu, L.; Pan, C.; Wang, S.; Li, F.; Wang, J. H.; Yue, H.; Gao, X. Y.; Jia, R. R. et al. Biomineralized bacterial outer membrane vesicles potentiate safe and efficient tumor microenvironment reprogramming for anticancer therapy. Adv. Mater. 2020, 32, e2002085.
Ban, W. Y.; Sun, M. C.; Huang, H. W.; Huang, W. X.; Pan, S. W.; Liu, P. F.; Li, B. W.; Cheng, Z. G.; He, Z. G.; Liu, F. N. et al. Engineered bacterial outer membrane vesicles encapsulating oncolytic adenoviruses enhance the efficacy of cancer virotherapy by augmenting tumor cell autophagy. Nat. Commun. 2023, 14, 2933.
Guo, Q.; Li, X. W.; Zhou, W. X.; Chu, Y. C.; Chen, Q. J.; Zhang, Y. W.; Li, C.; Chen, H. Y.; Liu, P. X.; Zhao, Z. H. et al. Sequentially triggered bacterial outer membrane vesicles for macrophage metabolism modulation and tumor metastasis suppression. ACS Nano 2021, 15, 13826–13838.
Vanaja, S. K.; Russo, A. J.; Behl, B.; Banerjee, I.; Yankova, M.; Deshmukh, S. D.; Rathinam, V. A. K. Bacterial outer membrane vesicles mediate cytosolic localization of LPS and caspase-11 activation. Cell 2016, 165, 1106–1119.
Li, Y. J.; Wu, J. Y.; Qiu, X. H.; Dong, S. H.; He, J.; Liu, J. H.; Xu, W. J.; Huang, S.; Hu, X. B.; Xiang, D. X. Bacterial outer membrane vesicles-based therapeutic platform eradicates triple-negative breast tumor by combinational photodynamic/chemo-/immunotherapy. Bioact. Mater. 2023, 20, 548–560.
Pfalzgraff, A.; Correa, W.; Heinbockel, L.; Schromm, A. B.; Lübow, C.; Gisch, N.; Martinez-de-Tejada, G.; Brandenburg, K.; Weindl, G. LPS-neutralizing peptides reduce outer membrane vesicle-induced inflammatory responses. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 1503–1513.
Li, Y.; Ma, X. T.; Yue, Y. L.; Zhang, K. Y.; Cheng, K. M.; Feng, Q. Q.; Ma, N. N.; Liang, J.; Zhang, T. J.; Zhang, L. Z. et al. Rapid surface display of mRNA antigens by bacteria-derived outer membrane vesicles for a personalized tumor vaccine. Adv. Mater. 2022, 34, 2109984.
Gujrati, V.; Kim, S.; Kim, S. H.; Min, J. J.; Choy, H. E.; Kim, S. C.; Jon, S. Bioengineered bacterial outer membrane vesicles as cell-specific drug-delivery vehicles for cancer therapy. ACS Nano 2014, 8, 1525–1537.
Aktar, S.; Okamoto, Y.; Ueno, S.; Tahara, Y. O.; Imaizumi, M.; Shintani, M.; Miyata, M.; Futamata, H.; Nojiri, H.; Tashiro, Y. Incorporation of plasmid DNA into bacterial membrane vesicles by peptidoglycan defects in Escherichia coli. Front. Microbiol. 2021, 12, 747606.
Wang, S. H.; Gao, J.; Wang, Z. J. Outer membrane vesicles for vaccination and targeted drug delivery. WIREs Nanomed. Nanobiotechnol. 2019, 11, e1523.
Li, M.; Zhou, H.; Yang, C.; Wu, Y.; Zhou, X. C.; Liu, H.; Wang, Y. C. Bacterial outer membrane vesicles as a platform for biomedical applications: An update. J. Control. Release 2020, 323, 253–268.
Swift, L. P.; Rephaeli, A.; Nudelman, A.; Phillips, D. R.; Cutts, S. M. Doxorubicin-DNA adducts induce a non-topoisomerase II-mediated form of cell death. Cancer Res. 2006, 66, 4863–4871.
Kuerban, K.; Gao, X. W.; Zhang, H.; Liu, J. Y.; Dong, M. X.; Wu, L. N.; Ye, R. H.; Feng, M. Q.; Ye, L. Doxorubicin-loaded bacterial outer-membrane vesicles exert enhanced anti-tumor efficacy in non-small-cell lung cancer. Acta Pharm. Sin. B 2020, 10, 1534–1548.
Mirzaei-Kalar, Z.; Nejad, Z. K.; Khandar, A. A. New ZnFe2O4@SiO2@graphene quantum dots as an effective nanocarrier for targeted DOX delivery and CT-DNA binder. J. Mol. Liq. 2022, 363, 119904.
Zhang, L.; Liao, W. Q.; Chen, S. M.; Chen, Y. K.; Cheng, P. R.; Lu, X. J.; Ma, Y. Towards a New 3Rs Era in the construction of 3D cell culture models simulating tumor microenvironment. Front. Oncol. 2023, 13, 1146477.
Fontana, F.; Marzagalli, M.; Sommariva, M.; Gagliano, N.; Limonta, P. In vitro 3D cultures to model the tumor microenvironment. Cancers (Basel) 2021, 13, 2970.
Kievit, F. M.; Florczyk, S. J.; Leung, M. C.; Veiseh, O.; Park, J. O.; Disis, M. L.; Zhang, M. Q. Chitosan-alginate 3D scaffolds as a mimic of the glioma tumor microenvironment. Biomaterials 2010, 31, 5903–5910.
Curvello, R.; Kast, V.; Abuwarwar, M. H.; Fletcher, A. L.; Garnier, G.; Loessner, D. 3D collagen-nanocellulose matrices model the tumour microenvironment of pancreatic cancer. Front. Digit. Health 2021, 3, 704584.
Dai, H. X.; Yang, Q. Y.; Sun, R.; Zhang, Y.; Ma, Q. L.; Shen, Y. F.; Wang, B. L.; Chen, Y. T.; Xu, J. L.; Tian, B. et al. Nanoparticle accumulation in liver may induce resistance to immune checkpoint blockade therapy. Nano Res. 2023, 16, 5237–5246.
Le-Wendling, L.; Nin, O.; Capdevila, X. Cancer recurrence and regional anesthesia: The theories, the data, and the future in outcomes. Pain Med. 2016, 17, 756–775.
Cakmakkaya, O. S.; Kolodzie, K.; Apfel, C. C.; Pace, N. L. Anaesthetic techniques for risk of malignant tumour recurrence. Cochrane Database Syst. Rev. 2014, CD008877.
Aguirre-Ghiso, J. A. Models, mechanisms and clinical evidence for cancer dormancy. Nat. Rev. Cancer 2007, 7, 834–846.
Xu, B. L.; Cui, Y.; Wang, W. W.; Li, S. S.; Lyu, C.; Wang, S.; Bao, W. E.; Wang, H. Y.; Qin, M.; Liu, Z. et al. Immunomodulation-enhanced nanozyme-based tumor catalytic therapy. Adv Mater. 2020, 32, e2003563.
Acknowledgements
This work was supported by the Beijing Natural Science Foundation (No. JQ21027) and the National Natural Science Foundation of China (Nos. U2001224, 32030062, 21821005, and 82202028).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Li, S., Li, X., Meng, J. et al. Dynamic shielding of bacterial outer membrane vesicles for safe and efficient chemo-immunotherapy against tumors. Nano Res. 17, 836–847 (2024). https://doi.org/10.1007/s12274-023-6225-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-6225-6