Abstract
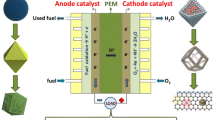
To achieve the goals of the peak carbon dioxide emissions and carbon neutral, the development and utilization of sustainable clean energy are extremely important. Hydrogen fuel cells are an important system for converting hydrogen energy into electrical energy. However, the slow hydrogen oxidation reaction (HOR) kinetics under alkaline conditions has limited its development. Therefore, elucidating the catalytic mechanism of HOR in acidic and alkaline media is of great significance for the construction of highly active and stable catalysts. In terms of practicality, Pt is still the primary choice for commercialization of fuel cells. On the above basis, we first introduced the hydrogen binding energy theory and bifunctional theory used to describe the HOR activity, as well as the pH dependence. After that, the rational design strategies of Pt-based HOR catalysts were systematically classified and summarized from the perspective of activity descriptors. In addition, we further emphasized the importance of theoretical simulations and in situ characterization in revealing the HOR mechanism, which is crucial for the rational design of catalysts. Moreover, the practical application of Pt-based HOR catalysts in fuel cells was also presented. In closing, the current challenges and future development directions of HOR catalysts were discussed. This review will provide a deep understanding for exploring the mechanism of highly efficient HOR catalysts and the development of fuel cells.

Similar content being viewed by others
References
Adabi, H.; Shakouri, A.; Ul Hassan, N.; Varcoe, J. R.; Zulevi, B.; Serov, A.; Regalbuto, J. R.; Mustain, W. E. High-performing commercial Fe-N-C cathode electrocatalyst for anion-exchange membrane fuel cells. Nat. Energy 2021, 6, 834–843.
Zhang, X.; Yu, P.; Xing, G. Y.; Xie, Y.; Zhang, X. X.; Zhang, G. Y.; Sun, F. F.; Wang, L. Iron single atoms-assisted cobalt nitride nanoparticles to strengthen the cycle life of rechargeable Zn-air battery. Small 2022, 18, 2205228.
Seh, Z. W.; Kibsgaard, J.; Dickens, C. F.; Chorkendorff, I.; Nørskov, J. K.; Jaramillo, T. F. Combining theory and experiment in electrocatalysis: Insights into materials design. Science 2017, 355, eaad4998.
Huang, L.; Zaman, S.; Wang, Z. T.; Niu, H. T.; You, B.; Xia, B. Y. Synthesis and application of platinum-based hollow nanoframes for direct alcohol fuel cells. Acta Phys. Chim. Sin. 2021, 37, 2009035.
Fu, X.; Li, N.; Ren, B.; Jiang, G.; Liu, Y.; Hassan, F. M.; Su, D.; Zhu, J.; Yang, L.; Bai, Z.; Cano, Z. P.; Yu, A.; Chen, Z. Tailoring FeN4 sites with edge enrichment for boosted oxygen reduction performance in proton exchange membrane fuel cell. Adv. Energy Mater. 2019, 9, 1803737.
Duan, X.; Cao, F.; Ding, R.; Li, X. K.; Li, Q. B.; Aisha, R.; Zhang, S. Q.; Hua, K.; Rui, Z. Y.; Wu, Y. K. et al. Cobalt-doping stabilized active and durable sub-2 nm Pt nanoclusters for low-Pt-loading PEMFC cathode. Adv. Energy Mater. 2022, 12, 2103144.
Xia, W.; Mahmood, A.; Liang, Z. B.; Zou, R. Q.; Guo, S. J. Earth-abundant nanomaterials for oxygen reduction. Angew. Chem., Int. Ed. 2016, 55, 2650–2676.
Xu, W. J.; Sun, Y. D.; Zhou, J. Q.; Cao, M. Q.; Luo, J.; Mao, H. L.; Hu, P. F.; Gu, H. F.; Zhai, H. Z.; Shang, H. et al. Coordinatively unsaturated single Co atoms immobilized on C2N for efficient oxygen reduction reaction. Nano Res. 2023, 16, 2294–2301.
Niu, H. T.; Xia, C. F.; Huang, L.; Zaman, S.; Maiyalagan, T.; Guo, W.; You, B.; Xia, B. Y. Rational design and synthesis of one-dimensional platinum-based nanostructures for oxygen-reduction electrocatalysis. Chin. J. Catal. 2022, 43, 1459–1472.
Setzler, B. P.; Zhuang, Z. B.; Wittkopf, J. A.; Yan, Y. S. Activity targets for nanostructured platinum-group-metal-free catalysts in hydroxide exchange membrane fuel cells. Nat. Nanotechnol. 2016, 11, 1020–1025.
Song, Z. X.; Banis, M. N.; Liu, H. S.; Zhang, L.; Zhao, Y.; Li, J. J.; Doyle-Davis, K.; Li, R. Y.; Knights, S.; Ye, S. Y. et al. Ultralow loading and high-performing Pt catalyst for a polymer electrolyte membrane fuel cell anode achieved by atomic layer deposition. ACS Catal. 2019, 9, 5365–5374.
Bhowmik, T.; Kundu, M. K.; Barman, S. Palladium nanoparticle-graphitic carbon nitride porous synergistic catalyst for hydrogen evolution/oxidation reactions over a broad range of pH and correlation of its catalytic activity with measured hydrogen binding energy. ACS Catal. 2016, 6, 1929–1941.
Zhou, Y. Y.; Xie, Z. Y.; Jiang, J. X.; Wang, J.; Song, X. Y.; He, Q.; Ding, W.; Wei, Z. D. Lattice-confined Ru clusters with high CO tolerance and activity for the hydrogen oxidation reaction. Nat. Catal. 2020, 3, 454–462.
Yang, X. L.; Wang, Y.; Wang, X.; Mei, B. B.; Luo, E. G.; Li, Y.; Meng, Q. L.; Jin, Z.; Jiang, Z.; Liu, C. P. et al. CO-tolerant PEMFC anodes enabled by synergistic catalysis between iridium single-atom sites and nanoparticles. Angew. Chem., Int. Ed. 2021, 60, 26177–26183.
Zhuang, Z. B.; Giles, S. A.; Zheng, J.; Jenness, G. R.; Caratzoulas, S.; Vlachos, D. G.; Yan, Y. S. Nickel supported on nitrogen-doped carbon nanotubes as hydrogen oxidation reaction catalyst in alkaline electrolyte. Nat. Commun. 2016, 7, 10141.
Zhan, C. H.; Xu, Y.; Bu, L. Z.; Zhu, H. Z.; Feng, Y. G.; Yang, T.; Zhang, Y.; Yang, Z. Q.; Huang, B. L.; Shao, Q. et al. Q. Subnanometer high-entropy alloy nanowires enable remarkable hydrogen oxidation catalysis. Nat. Commun. 2021, 12, 6261.
Duan, Y.; Zhang, X. L.; Gao, F. Y.; Kong, Y.; Duan, Y.; Yang, X. T.; Yu, X. X.; Wang, Y. R.; Qin, S.; Chen, Z. et al. Interfacial engineering of Ni/V2O3 heterostructure catalyst for boosting hydrogen oxidation reaction in alkaline electrolytes. Angew. Chem., Int. Ed. 2023, 62, e202217275.
Zhang, B. H.; Zhao, G. Q.; Zhang, B. X.; Xia, L. X.; Jiang, Y. Z.; Ma, T. Y.; Gao, M. X.; Sun, W. P.; Pan, H. G. Lattice-confined Ir clusters on Pd nanosheets with charge redistribution for the hydrogen oxidation reaction under alkaline conditions. Adv. Mater. 2021, 33, 2105400.
Sheng, W. C.; Gasteiger, H. A.; Shao-Horn, Y. Hydrogen oxidation and evolution reaction kinetics on platinum: Acid vs alkaline electrolytes. J. Electrochem. Soc. 2010, 157, B1529–B1536.
Thompson, S. T.; Peterson, D.; Ho, D.; Papageorgopoulos, D. Perspective—The next decade of AEMFCs: Near-term targets to accelerate applied R&D. J. Electrochem. Soc. 2020, 167, 084514.
Firouzjaie, H. A.; Mustain, W. E. Catalytic advantages, challenges, and priorities in alkaline membrane fuel cells. ACS Catal. 2020, 10, 225–234.
Lafforgue, C.; Chatenet, M.; Dubau, L.; Dekel, D. R. Accelerated stress test of Pt/C nanoparticles in an interface with an anion-exchange membrane—An identical-location transmission electron microscopy study. ACS Catal. 2018, 8, 1278–1286.
Zadick, A.; Dubau, L.; Sergent, N.; Berthome, G.; Chatenet, M. Huge instability of Pt/C catalysts in alkaline medium. ACS Catal. 2015, 5, 4819–4824.
Kitchin, J. R.; Nørskov, J. K.; Barteau, M. A.; Chen, J. G. Modification of the surface electronic and chemical properties of Pt(111) by subsurface 3d transition metals. J. Chem. Phys. 2004, 120, 10240–10246.
Lee, D. W.; Choi, D.; Lee, M. J.; Jin, H.; Lee, S.; Jang, I.; Park, H. Y.; Jang, J. H.; Kim, H. J.; Lee, K. Y. et al. Tailoring of Pt island RuO2/C catalysts by galvanic replacement to achieve superior hydrogen oxidation reaction and CO poisoning resistance. ACS Appl. Energy Mater. 2021, 4, 8098–8107.
Davydova, E. S.; Mukerjee, S.; Jaouen, F.; Dekel, D. R. Electrocatalysts for hydrogen oxidation reaction in alkaline electrolytes. ACS Catal. 2018, 8, 6665–6690.
Peng, L. X.; Tian, H.; Cui, X. Z.; Su, L.; Meng, G.; Ma, Z. H.; Cao, S. W.; Shi, J. L. Dual synergetic catalytic effects boost hydrogen electric oxidation performance of Pd/W18O49. Nano Res. 2021, 14, 2441–2450.
Sheng, W. C.; Myint, M.; Chen, J. G.; Yan, Y. S. Correlating the hydrogen evolution reaction activity in alkaline electrolytes with the hydrogen binding energy on monometallic surfaces. Energy Environ. Sci. 2013, 6, 1509–1512.
Angerstein-Kozlowska, H.; Conway, B. E.; Hamelin, A. Electrocatalytic mediation of oxidation of H2 at gold by chemisorbed states of anions. J. Electroanal. Chem. Interfac. Electrochem. 1990, 277, 233–252.
Strmcnik, D.; Uchimura, M.; Wang, C.; Subbaraman, R.; Danilovic, N.; Van Der Vliet, D.; Paulikas, A. P.; Stamenkovic, V. R.; Markovic, N. M. Improving the hydrogen oxidation reaction rate by promotion of hydroxyl adsorption. Nat. Chem. 2013, 5, 300–306.
Shinagawa, T.; Garcia-Esparza, A. T.; Takanabe, K. Insight on Tafel slopes from a microkinetic analysis of aqueous electrocatalysis for energy conversion. Sci. Rep. 2015, 5, 13801.
Gao, F. Y.; Wang, Y. H.; Yang, Y.; Liao, J.; Duanmu, J. W.; Zhang, X. L.; Niu, Z. Z.; Yang, P. P.; Gao, M. R. Towards reliable assessment of hydrogen oxidation electrocatalysts for anion-exchange membrane fuel cells. Nano Res., in press, https://doi.org/10.1007/s12274-023-5792-x.
St John, S.; Atkinson III, R. W.; Unocic, R. R.; Zawodzinski, T. A. Jr.; Papandrew, A. B. Ruthenium-alloy electrocatalysts with tunable hydrogen oxidation kinetics in alkaline electrolyte. J. Phys. Chem. C 2015, 119, 13481–13487.
Montero, M. A.; De Chialvo, M. R. G.; Chialvo, A. C. Kinetics of the hydrogen oxidation reaction on nanostructured rhodium electrodes in alkaline solution. J. Power Sources 2015, 283, 181–186.
Montero, M. A.; De Chialvo, M. R. G.; Chialvo, A. C. Evaluation of the kinetic parameters of the hydrogen oxidation reaction on nanostructured iridium electrodes in alkaline solution. J. Electroanal. Chem. 2016, 167, 153–159.
Li, J. K.; Ghoshal, S.; Bates, M. K.; Miller, T. E.; Davies, V.; Stavitski, E.; Attenkofer, K.; Mukerjee, S.; Ma, Z. F.; Jia, Q. Y. Experimental proof of the bifunctional mechanism for the hydrogen oxidation in alkaline media. Angew. Chem., Int. Ed. 2017, 56, 15594–15598.
Elbert, K.; Hu, J.; Ma, Z.; Zhang, Y.; Chen, G. Y.; An, W.; Liu, P.; Isaacs, H. S.; Adzic, R. R.; Wang, J. X. Elucidating hydrogen oxidation/evolution kinetics in base and acid by enhanced activities at the optimized Pt shell thickness on the Ru core. ACS Catal. 2015, 5, 6764–6772.
Luo, H.; Wang, K.; Lin, F. X.; Lv, F.; Zhou, J. H.; Zhang, W. Y.; Wang, D. W.; Zhang, W. S.; Zhang, Q. H.; Gu, L. et al. Amorphous MoOx with high oxophilicity interfaced with PtMo alloy nanoparticles boosts anti-CO hydrogen electrocatalysis. Adv. Mater., in press, https://doi.org/10.1002/adma.202211854.
Zhao, T. H.; Hu, Y. C.; Gong, M. X.; Lin, R. Q.; Deng, S. F.; Lu, Y.; Liu, X. P.; Chen, Y.; Shen, T.; Hu, Y. Z. et al. Electronic structure and oxophilicity optimization of mono-layer Pt for efficient electrocatalysis. Nano Energy 2020, 74, 104877.
Yang, F. L.; Bao, X.; Li, P.; Wang, X. W.; Cheng, G. Z.; Chen, S. L.; Luo, W. Boosting hydrogen oxidation activity of Ni in alkaline media through oxygen-vacancy-rich CeO2/Ni heterostructures. Angew. Chem., Int. Ed. 2019, 58, 14179–14183.
Qiu, Y.; Xin, L.; Li, Y. W.; McCrum, I. T.; Guo, F. M.; Ma, T.; Ren, Y.; Liu, Q.; Zhou, L.; Gu, S. et al. BCC-phased PdCu alloy as a highly active electrocatalyst for hydrogen oxidation in alkaline electrolytes. J. Am. Chem. Soc. 2018, 140, 16580–16588.
Rheinländer, P. J.; Herranz, J.; Durst, J.; Gasteiger, H. A. Kinetics of the hydrogen oxidation/evolution reaction on polycrystalline platinum in alkaline electrolyte reaction order with respect to hydrogen pressure. J. Electrochem. Soc. 2014, 161, F1448–F1457.
Zheng, J.; Yan, Y. S.; Xu, B. J. Correcting the hydrogen diffusion limitation in rotating disk electrode measurements of hydrogen evolution reaction kinetics. J. Electrochem. Soc. 2015, 162, F1470–F1481.
Durst, J.; Simon, C.; Hasche, F.; Gasteiger, H. A. Hydrogen oxidation and evolution reaction kinetics on carbon supported Pt, Ir, Rh, and Pd electrocatalysts in acidic media. J. Electrochem. Soc. 2015, 162, F190–F203.
Chen, S. L.; Kucernak, A. Electrocatalysis under conditions of high mass transport: Investigation of hydrogen oxidation on single submicron Pt particles supported on carbon. J. Phys. Chem. B 2004, 108, 13984–13994.
Zoski, C. G. Scanning electrochemical microscopy: Investigation of hydrogen oxidation at polycrystalline noble metal electrodes. J. Phys. Chem. B 2003, 107, 6401–6405.
Zalitis, C. M.; Kramer, D.; Kucernak, A. R. Electrocatalytic performance of fuel cell reactions at low catalyst loading and high mass transport. Phys. Chem. Chem. Phys. 2013, 15, 4329–4340.
Zhao, R. P.; Yue, X.; Li, Q. H.; Fu, G. T.; Lee, J. M.; Huang, S. M. Recent advances in electrocatalysts for alkaline hydrogen oxidation reaction. Small 2021, 17, 2100391.
Xiao, F.; Wang, Y. C.; Wu, Z. P.; Chen, G. Y.; Yang, F.; Zhu, S. Q.; Siddharth, K.; Kong, Z. J.; Lu, A. L.; Li, J. C. et al. Recent advances in electrocatalysts for proton exchange membrane fuel cells and alkaline membrane fuel cells. Adv. Mater. 2021, 33, 2006292.
Medford, A. J.; Vojvodic, A.; Hummelshøj, J. S.; Voss, J.; Abild-Pedersen, F.; Studt, F.; Bligaard, T.; Nilsson, A.; Nørskov, J. K. From the Sabatier principle to a predictive theory of transitionmetal heterogeneous catalysis. J. Catal. 2015, 328, 36–42.
Zhang, H.; Wang, J.; Qin, F. Q.; Liu, H. L.; Wang, C. V-doped Ni3N/Ni heterostructure with engineered interfaces as a bifunctional hydrogen electrocatalyst in alkaline solution: Simultaneously improving water dissociation and hydrogen adsorption. Nano Res. 2021, 14, 3489–3496.
Nørskov, J. K.; Bligaard, T.; Logadottir, A.; Kitchin, J. R.; Chen, J. G.; Pandelov, S.; Stimming, U. Trends in the exchange current for hydrogen evolution. J. Electrochem. Soc. 2005, 152, J23–J26.
Zheng, J.; Sheng, W. C.; Zhuang, Z. B.; Xu, B. J.; Yan, Y. S. Universal dependence of hydrogen oxidation and evolution reaction activity of platinum-group metals on pH and hydrogen binding energy. Sci. Adv. 2016, 2, e1501602.
Sheng, W. C.; Zhuang, Z. B.; Gao, M. R.; Zheng, J.; Chen, J. G.; Yan, Y. S. Correlating hydrogen oxidation and evolution activity on platinum at different pH with measured hydrogen binding energy. Nat. Commun. 2015, 6, 5848.
Zheng, J.; Zhuang, Z. B.; Xu, B. J.; Yan, Y. S. Correlating hydrogen oxidation/evolution reaction activity with the minority weak hydrogen-binding sites on Ir/C catalysts. ACS Catal. 2015, 5, 4449–4455.
Sheng, W. C.; Bivens, A. P.; Myint, M.; Zhuang, Z. B.; Forest, R. V.; Fang, Q. R.; Chen, J. G.; Yan, Y. S. Non-precious metal electrocatalysts with high activity for hydrogen oxidation reaction in alkaline electrolytes. Energy Environ. Sci. 2014, 7, 1719–1724.
Ghoshal, S.; Jia, Q. Y.; Bates, M. K.; Li, J. K.; Xu, C. C.; Gath, K.; Yang, J.; Waldecker, J.; Che, H. Y.; Liang, W. T. et al. Tuning Nb-Pt interactions to facilitate fuel cell electrocatalysis. ACS Catal. 2017, 7, 4936–4946.
Ni, W. Y.; Wang, T.; Héroguel, F.; Krammer, A.; Lee, S.; Yao, L.; Schüler, A.; Luterbacher, J. S.; Yan, Y. S.; Hu, X. L. An efficient nickel hydrogen oxidation catalyst for hydroxide exchange membrane fuel cells. Nat. Mater. 2022, 21, 804–810.
Wang, K. C.; Yang, H.; Zhang, J. T.; Ren, G. M.; Cheng, T.; Xu, Y.; Huang, X. Q. The exclusive surface and electronic effects of Ni on promoting the activity of Pt towards alkaline hydrogen oxidation. Nano Res. 2022, 15, 5865–5872.
McCrum, I. T.; Koper, M. T. M. The role of adsorbed hydroxide in hydrogen evolution reaction kinetics on modified platinum. Nat. Energy 2020, 5, 891–899.
Jiang, J. X.; Tao, S. C.; He, Q.; Wang, J.; Zhou, Y. Y.; Xie, Z. Y.; Ding, W.; Wei, Z. D. Interphase-oxidized ruthenium metal with half-filled d-orbitals for hydrogen oxidation in an alkaline solution. J. Mater. Chem. A 2020, 8, 10168–10174.
Ledezma-Yanez, I.; Wallace, W. D. Z.; Sebastián-Pascual, P.; Climent, V.; Feliu, J. M.; Koper, M. T. M. Interfacial water reorganization as a pH-dependent descriptor of the hydrogen evolution rate on platinum electrodes. Nat. Energy 2017, 2, 17031.
Mu, X. Q.; Liu, S. L.; Chen, L.; Mu, S. C. Alkaline hydrogen oxidation reaction catalysts: Insight into catalytic mechanisms, classification, activity regulation and challenges. Small Struct. 2023, 4, 2200281.
Giles, S. A.; Wilson, J. C.; Nash, J.; Xu, B. J.; Vlachos, D. G.; Yan, Y. S. Recent advances in understanding the pH dependence of the hydrogen oxidation and evolution reactions. J. Catal. 2018, 367, 328–331.
Tian, X. Y.; Zhao, P. C.; Sheng, W. C. Hydrogen evolution and oxidation: Mechanistic studies and material advances. Adv. Mater. 2019, 31, 1808066.
Durst, J.; Siebel, A.; Simon, C.; Hasché, F.; Herranz, J.; Gasteiger, H. A. New insights into the electrochemical hydrogen oxidation and evolution reaction mechanism. Energy Environ. Sci. 2014, 7, 2255–2260.
Schouten, K. J. P.; Van Der Niet, M. J. T. C.; Koper, M. T. M. Impedance spectroscopy of H and OH adsorption on stepped single-crystal platinum electrodes in alkaline and acidic media. Phys. Chem. Chem. Phys. 2010, 12, 15217–15224.
Li, M. T.; Li, L.; Huang, X.; Qi, X. Q.; Deng, M. M.; Jiang, S. K.; Wei, Z. D. Platinum-water interaction induced interfacial water orientation that governs the pH-dependent hydrogen oxidation reaction. J. Phys. Chem. Lett. 2022, 13, 10550–10557.
Shen, L. F.; Lu, B. A.; Li, Y. Y.; Liu, J.; Huangfu, Z. C.; Peng, H.; Ye, J. Y.; Qu, X. M.; Zhang, J. M.; Li, G. et al. Interfacial structure of water as a new descriptor of the hydrogen evolution reaction. Angew. Chem., Int. Ed. 2020, 59, 22397–22402.
Karlberg, G. S.; Jaramillo, T. F.; Skúlason, E.; Rossmeisl, J.; Bligaard, T.; Nørskov, J. K. Cyclic voltammograms for H on Pt(111) and Pt(100) from first principles. Phys. Rev. Lett. 2007, 99, 126101.
Wang, Y. H.; Zheng, S. S.; Yang, W. M.; Zhou, R. Y.; He, Q. F.; Radjenovic, P.; Dong, J. C.; Li, S. N.; Zheng, J. X.; Yang, Z. L. et al. In situ Raman spectroscopy reveals the structure and dissociation of interfacial water. Nature 2021, 600, 81–85.
Zheng, J.; Nash, J.; Xu, B. J.; Yan, Y. S. Perspective-towards establishing apparent hydrogen binding energy as the descriptor for hydrogen oxidation/evolution reactions. J. Electrochem. Soc. 2018, 165, H27–H29.
Cheng, T.; Wang, L.; Merinov, B. V.; Goddard, W. A. Explanation of dramatic pH-dependence of hydrogen binding on noble metal electrode: Greatly weakened water adsorption at high pH. J. Am. Chem. Soc. 2018, 140, 7787–7790.
Li, P.; Jiang, Y. L.; Hu, Y. C.; Men, Y. N.; Liu, Y. W.; Cai, W. B.; Chen, S. L. Hydrogen bond network connectivity in the electric double layer dominates the kinetic pH effect in hydrogen electrocatalysis on Pt. Nat. Catal. 2022, 5, 900–911.
Dong, Y. T.; Sun, Q. T.; Zhan, C. H.; Zhang, J. T.; Yang, H.; Cheng, T.; Xu, Y.; Hu, Z. W.; Pao, C. W.; Geng, H. B. et al. Lattice and surface engineering of ruthenium nanostructures for enhanced hydrogen oxidation catalysis. Adv. Funct. Mater. 2023, 33, 2210328.
Li, L. G.; Liu, S. H.; Zhan, C. H.; Wen, Y.; Sun, Z. F.; Han, J. J.; Chan, T. S.; Zhang, Q. B.; Hu, Z. W.; Huang, X. Q. Surface and lattice engineered ruthenium superstructures towards high-performance bifunctional hydrogen catalysis. Energy Environ. Sci. 2023, 16, 157–166.
Yan, B.; Bisbey, R. P.; Alabugin, A.; Surendranath, Y. Mixed electron-proton conductors enable spatial separation of bond activation and charge transfer in electrocatalysis. J. Am. Chem. Soc. 2019, 141, 11115–11122.
Xiao, W. P.; Lei, W.; Wang, J.; Gao, G. Y.; Zhao, T. H.; Cordeiro, M. A. L.; Lin, R. Q.; Gong, M. X.; Guo, X. Y.; Stavitski, E. et al. Tuning the electrocatalytic activity of Pt by structurally ordered PdFe/C for the hydrogen oxidation reaction in alkaline media. J. Mater. Chem. A 2018, 6, 11346–11352.
Zhao, T. H.; Wang, G. J.; Gong, M. X.; Xiao, D. D.; Chen, Y.; Shen, T.; Lu, Y.; Zhang, J.; Xin, H. L.; Li, Q. et al. Self-optimized ligand effect in L12-PtPdFe intermetallic for efficient and stable alkaline hydrogen oxidation reaction. ACS Catal. 2020, 10, 15207–15216.
Cong, Y. Y.; Yi, B. L.; Song, Y. J. Hydrogen oxidation reaction in alkaline media: From mechanism to recent electrocatalysts. Nano Energy 2018, 44, 288–303.
Zhang, Y. W.; Chen, T.; Alia, S.; Pivovar, B. S.; Xu, W. L. Single-molecule nanocatalysis shows in situ deactivation of Pt/C electrocatalysts during the hydrogen-oxidation reaction. Angew. Chem., Int. Ed. 2016, 128, 3138–3142.
Song, J. D.; Jin, Y. Q.; Zhang, L.; Dong, P. Y.; Li, J. W.; Xie, F. Y.; Zhang, H.; Chen, J.; Jin, Y. S.; Meng, H. et al. Phase-separated Mo-Ni alloy for hydrogen oxidation and evolution reactions with high activity and enhanced stability. Adv. Energy Mater. 2021, 11, 2003511.
Zhao, T. H.; Li, M. T.; Xiao, D. D.; Yang, X. J.; Li, Q. H.; An, L. L.; Deng, Z. P.; Shen, T.; Gong, M. X.; Chen, Y. et al. Pseudo-Pt monolayer for robust hydrogen oxidation. J. Am. Chem. Soc. 2023, 145, 4088–4097.
Ma, S. Y.; Ma, T.; Hu, Q.; Yang, H. P.; He, C. X. Ternary PtRuTe alloy nanofibers as an efficient and durable electrocatalyst for hydrogen oxidation reaction in alkaline media. Sci. China Mater. 2022, 65, 3462–3469.
Wang, M. M.; Wang, M. J.; Zhan, C. H.; Geng, H. B.; Li, Y. H.; Huang, X. Q.; Bu, L. Z. Ultrafine platinum-iridium distorted nanowires as robust catalysts toward bifunctional hydrogen catalysis. J. Mater. Chem. A 2022, 10, 18972–18977.
Liao, H. G.; Jiang, Y. X.; Zhou, Z. Y.; Chen, S. P.; Sun, S. G. Shape-controlled synthesis of gold nanoparticles in deep eutectic solvents for studies of structure-functionality relationships in electrocatalysis. Angew. Chem., Int. Ed. 2008, 120, 9240–9243.
Liu, S.; Tian, N.; Xie, A. Y.; Du, J. H.; Xiao, J.; Liu, L.; Sun, H. Y.; Cheng, Z. Y.; Zhou, Z. Y.; Sun, S. G. Electrochemically seed-mediated synthesis of sub-10 nm tetrahexahedral Pt nanocrystals supported on graphene with improved catalytic performance. J. Am. Chem. Soc. 2016, 138, 5753–5756.
Hoshi, N.; Asaumi, Y.; Nakamura, M.; Mikita, K.; Kajiwara, R. Structural effects on the hydrogen oxidation reaction on n(111)–(111) surfaces of platinum. J. Phys. Chem. C 2009, 113, 16843–16846.
Wang, N.; Wang, D. X.; Wu, A. P.; Wang, S. Y.; Li, Z. H.; Jin, C. X.; Dong, Y. M.; Kong, F. Y.; Tian, C. G.; Fu, H. G. Few-layered MoS2 anchored on 2D porous C3N4 nanosheets for Pt-free photocatalytic hydrogen evolution. Nano Res. 2023, 16, 3524–3535.
De Luna, P.; Quintero-Bermudez, R.; Dinh, C. T.; Ross, M. B.; Bushuyev, O. S.; Todorović, P.; Regier, T.; Kelley, S. O.; Yang, P. D.; Sargent, E. H. Catalyst electro-redeposition controls morphology and oxidation state for selective carbon dioxide reduction. Nat. Catal. 2018, 1, 103–110.
Stephens, I. E. L.; Bondarenko, A. S.; Grønbjerg, U.; Rossmeisl, J.; Chorkendorff, I. Understanding the electrocatalysis of oxygen reduction on platinum and its alloys. Energy Environ. Sci. 2012, 5, 6744–6762.
Guo, Y.; Hou, B.; Cui, X. Z.; Liu, X. C.; Tong, X. L.; Yang, N. J. Pt atomic layers boosted hydrogen evolution reaction in nonacidic media. Adv. Energy Mater. 2022, 12, 2201548.
Schwämmlein, J. N.; Stühmeier, B. M.; Wagenbauer, K.; Dietz, H.; Tileli, V.; Gasteiger, H. A.; El-Sayed, H. A. Origin of superior HOR/HER activity of bimetallic Pt-Ru catalysts in alkaline media identified via Ru@Pt core–shell nanoparticles. J. Electrochem. Soc. 2018, 165, H229–H239.
Esposito, D. V.; Hunt, S. T.; Kimmel, Y. C.; Chen, J. G. A new class of electrocatalysts for hydrogen production from water electrolysis: Metal monolayers supported on low-cost transition metal carbides. J. Am. Chem. Soc. 2012, 134, 3025–3033.
Wang, L.; Mahoney, E. G.; Zhao, S.; Yang, B. L.; Chen, J. G. Low loadings of platinum on transition metal carbides for hydrogen oxidation and evolution reactions in alkaline electrolytes. Chem. Commun. 2016, 52, 3697–3700.
Mao, J. J.; Chen, W. X.; He, D. S.; Wan, J. W.; Pei, J. J.; Dong, J. C.; Wang, Y.; An, P. F.; Jin, Z.; Xing, W. et al. Design of ultrathin Pt-Mo-Ni nanowire catalysts for ethanol electrooxidation. Sci. Adv. 2017, 3, e1603068.
Alia, S. M.; Pivovar, B. S.; Yan, Y. S. Platinum-coated copper nanowires with high activity for hydrogen oxidation reaction in base. J. Am. Chem. Soc. 2013, 135, 13473–13478.
Li, X. Y.; Cai, W. Z.; Li, D. S.; Xu, J.; Tao, H. B.; Liu, B. Amorphous alloys for electrocatalysis: The significant role of the amorphous alloy structure. Nano Res. 2023, 16, 4277–4288.
Adabi, H.; Shakouri, A.; Zitolo, A.; Asset, T.; Khan, A.; Bohannon, J.; Chattot, R.; Williams, C.; Jaouen, F.; Regalbuto, J. R. et al. Multi-atom Pt and PtRu catalysts for high performance AEMFCs with ultra-low PGM content. Appl. Catal. B Environ. 2023, 325, 122375.
Wang, H.; Abruña, H. D. Rh and Rh alloy nanoparticles as highly active H2 oxidation catalysts for alkaline fuel cells. ACS Catal. 2019, 9, 5057–5062.
Zhao, L. M.; Liu, H. J.; Liu, Y. H.; Han, X. N.; Xu, J.; Xing, W.; Guo, W. Y. Mechanistic insights into the hydrogen oxidation reaction on PtNi alloys in alkaline media: A first-principles investigation. ACS Appl. Mater. Interfaces 2020, 12, 40248–40260.
Balbuena, P. B.; Altomare, D.; Vadlamani, N.; Bingi, S.; Agapito, L. A.; Seminario, J. M. Adsorption of O, OH, and H2O on Pt-based bimetallic clusters alloyed with Co, Cr, and Ni. J. Phys. Chem. A 2004, 108, 6378–6384.
Wang, Y.; Wang, G. W.; Li, G. W.; Huang, B.; Pan, J.; Liu, Q.; Han, J. J.; Xiao, L.; Lu, J. T.; Zhuang, L. Pt-Ru catalyzed hydrogen oxidation in alkaline media: Oxophilic effect or electronic effect. Energy Environ. Sci. 2015, 8, 177–181.
Wang, G. W.; Li, W. Z.; Wu, N.; Huang, B.; Xiao, L.; Lu, J. T.; Zhuang, L. Unraveling the composition-activity relationship of Pt-Ru binary alloy for hydrogen oxidation reaction in alkaline media. J. Power Sources 2019, 412, 282–286.
Lu, S. Q.; Zhuang, Z. B. Investigating the influences of the adsorbed species on catalytic activity for hydrogen oxidation reaction in alkaline electrolyte. J. Am. Chem. Soc. 2017, 139, 5156–5163.
Weber, D. J.; Dosche, C.; Oezaslan, M. Tuning of Pt-Co nanoparticle motifs for enhancing the HOR performance in alkaline media. J. Mater. Chem. A 2021, 9, 15415–15431.
Yao, Z. C.; Tang, T.; Jiang, Z.; Wang, L.; Hu, J. S.; Wan, L. J. Electrocatalytic hydrogen oxidation in alkaline media: From mechanistic insights to catalyst design. ACS Nano 2022, 16, 5153–5183.
Scofield, M. E.; Zhou, Y. C.; Yue, S. Y.; Wang, L.; Su, D.; Tong, X.; Vukmirovic, M. B.; Adzic, R. R.; Wong, S. S. Role of chemical composition in the enhanced catalytic activity of Pt-based alloyed ultrathin nanowires for the hydrogen oxidation reaction under alkaline conditions. ACS Catal. 2016, 6, 3895–3908.
Cong, Y. Y.; Chai, C. X.; Zhao, X. W.; Yi, B. L.; Song Y. J. Pt0.25Ru0.75/N-C as highly active and durable electrocatalysts toward alkaline hydrogen oxidation reaction. Adv. Mater. Interfaces 2020, 7, 2000310.
Sun, Y. J.; Zhang, W. S.; Zhang, Q. H.; Li, Y. J.; Gu, L.; Guo, S. J. A general approach to high-entropy metallic nanowire electrocatalysts. Matter 2023, 6, 193–205.
Yang, J. R.; Li, W. H.; Wang, D. S.; Li, Y. D. Electronic metal-support interaction of single-atom catalysts and applications in electrocatalysis. Adv. Mater. 2020, 32, 2003300.
Xu, S.; Niu, M.; Zhao, G. W.; Ming, S. J.; Li, X. Y.; Zhu, Q. L.; Ding, L. X.; Kim, M.; Alothman, A. A.; Mushab, M. S. S. et al. Size control and electronic manipulation of Ru catalyst over B, N co-doped carbon network for high-performance hydrogen evolution reaction. Nano Res. 2023, 16, 6212–6219.
Zhang, X.; Wang, L. Research progress of carbon nanofiber-based precious-metal-free oxygen reaction catalysts synthesized by electrospinning for Zn-air batteries. J. Power Sources 2021, 507, 230280.
Gan, T.; Wang, D. S. Atomically dispersed materials: Ideal catalysts in atomic era. Nano Res., https://doi.org/10.1007/s12274-023-5700-4.
Liu, L. C.; Corma, A. Metal catalysts for heterogeneous catalysis: From single atoms to nanoclusters and nanoparticles. Chem. Rev. 2018, 118, 4981–5079.
Ming, M.; Zhang, Y.; He, C.; Zhao, L.; Niu, S.; Fan, G. Y.; Hu, J. S. Room-temperature sustainable synthesis of selected platinum group metal (PGM = Ir, Rh, and Ru) nanocatalysts well-dispersed on porous carbon for efficient hydrogen evolution and oxidation. Small 2019, 15, 1903057.
Wang, H. W.; Gu, X. K.; Zheng, X. S.; Pan, H. B.; Zhu, J. F.; Chen, S.; Cao, L. N.; Li, W. X.; Lu, J. L. Disentangling the size-dependent geometric and electronic effects of palladium nanocatalysts beyond selectivity. Sci. Adv. 2019, 5, eaat6413.
Han, B. C.; Miranda, C. R.; Ceder, G. Effect of particle size and surface structure on adsorption of O and OH on platinum nanoparticles: A first-principles study. Phys. Rev. B 2008, 77, 075410.
Sun, Y. B.; Zhuang, L.; Lu, J. T.; Hong, X. L.; Liu, P. F. Collapse in crystalline structure and decline in catalytic activity of Pt nanoparticles on reducing particle size to 1 nm. J. Am. Chem. Soc. 2007, 129, 15465–15467.
Wang, Z. P.; Pan, X. X.; Qian, S. Y.; Yang, G.; Du, F. L.; Yuan, X. The beauty of binary phases: A facile strategy for synthesis, processing, functionalization, and application of ultrasmall metal nanoclusters. Coord. Chem. Rev. 2021, 438, 213900.
Yuan, X.; Chng, L. L.; Yang, J. H.; Ying, J. Y. Miscible-solvent-assisted two-phase synthesis of monolayer-ligand-protected metal nanoclusters with various sizes. Adv. Mater. 2020, 32, 1906063.
Wang, X. N.; Zhao, L. M.; Li, X. J.; Liu, Y.; Wang, Y. S.; Yao, Q. F.; Xie, J. P.; Xue, Q. Z.; Yan, Z. F.; Yuan, X. et al. Atomic-precision Pt6 nanoclusters for enhanced hydrogen electro-oxidation. Nat. Commun. 2022, 13, 1596.
Yang, Z. J.; Chen, C. Q.; Zhao, Y. X.; Wang, Q.; Zhao, J. Q.; Waterhouse, G. I. N.; Qin, Y.; Shang, L.; Zhang, T. R. Pt single atoms on CrN nanoparticles deliver outstanding activity and CO tolerance in the hydrogen oxidation reaction. Adv. Mater. 2023, 35, 2208799.
Li, M. T.; Xie, Z. Y.; Zheng, X. Q.; Li, L.; Li, J.; Ding, W.; Wei, Z. D. Revealing the regulation mechanism of Ir-MoO2 interfacial chemical bonding for improving hydrogen oxidation reaction. ACS Catal. 2021, 11, 14932–14940.
Wang, T. Y.; Xie, H.; Chen, M. J.; D’Aloia, A.; Cho, J.; Wu, G.; Li, Q. Precious metal-free approach to hydrogen electrocatalysis for energy conversion: From mechanism understanding to catalyst design. Nano Energy 2017, 42, 69–89.
Zhao, G. Q.; Chen, J.; Sun, W. P.; Pan, H. G. Non-platinum group metal electrocatalysts toward efficient hydrogen oxidation reaction. Adv. Funct. Mater. 2021, 31, 2010633.
Feng, Z. P.; Li, L.; Zheng, X. Q.; Li, J.; Yang, N.; Ding, W.; Wei, Z. D. Role of hydroxyl species in hydrogen oxidation reaction: A DFT study. J. Phys. Chem. C 2019, 123, 23931–23939.
Liu, L.; Liu, Y. Y.; Liu, C. G. Enhancing the understanding of hydrogen evolution and oxidation reactions on Pt(111) through ab initio simulation of electrode/electrolyte kinetics. J. Am. Chem. Soc. 2020, 142, 4985–4989.
Skúlason, E.; Tripkovic, V.; Björketun, M. E.; Gudmundsdóttir, S.; Karlberg, G.; Rossmeisl, J.; Bligaard, T.; Jónsson, H.; Nørskov, J. K. Modeling the electrochemical hydrogen oxidation and evolution reactions on the basis of density functional theory calculations. J. Phys. Chem. C 2010, 114, 18182–18197.
Xue, W. J.; Liu, H. X.; Chen, X. Q.; Yang, X. J.; Yang, R. Q.; Liu, Y. W.; Li, M. H.; Yang, X.; Xia, B. Y.; You, B. Operando reconstruction towards stable CuI nanodots with favorable facets for selective CO2 electroreduction to C2H4. Sci. China Chem. 2023, 66, 1834–1843.
Chen, H. Q.; Zou, L.; Wei, D. Y.; Zheng, L. L.; Wu, Y. F.; Zhang, H.; Li, J. F. In situ studies of energy-related electrochemical reactions using Raman and X-ray absorption spectroscopy. Chin. J. Catal. 2022, 43, 33–46.
Li, J. F.; Huang, Y. F.; Ding, Y.; Yang, Z. L.; Li, S. B.; Zhou, X. S.; Fan, F. R.; Zhang, W.; Zhou, Z. Y.; Wu, D. Y. et al. Shell-isolated nanoparticle-enhanced Raman spectroscopy. Nature 2010, 464, 392–395.
Li, J. F.; Zhang, Y. J.; Ding, S. Y.; Panneerselvam, R.; Tian, Z. Q. Core-shell nanoparticle-enhanced Raman spectroscopy. Chem. Rev. 2017, 117, 5002–5069.
Wang, Y. H.; Wang, X. T.; Ze, H.; Zhang, X. G.; Radjenovic, P. M.; Zhang, Y. J.; Dong, J. C.; Tian, Z. Q.; Li, J. F. Spectroscopic verification of adsorbed hydroxy intermediates in the bifunctional mechanism of the hydrogen oxidation reaction. Angew. Chem., Int. Ed. 2021, 60, 5708–5711.
Lin, X. M.; Wang, X. T.; Deng, Y. L.; Chen, X.; Chen, H. N.; Radjenovic, P. M.; Zhang, X. G.; Wang, Y. H.; Dong, J. C.; Tian, Z. Q. et al. In situ probe of the hydrogen oxidation reaction intermediates on PtRu a bimetallic catalyst surface by core–shell nanoparticle-enhanced Raman spectroscopy. Nano Lett. 2022, 22, 5544–5552.
Li, J. K.; Gong, J. L. Operando characterization techniques for electrocatalysis. Energy Environ. Sci. 2020, 13, 3748–3779.
Zhu, S. Q.; Qin, X. P.; Xiao, F.; Yang, S. L.; Xu, Y.; Tan, Z.; Li, J. D.; Yan, J. W.; Chen, Q.; Chen, M. S. et al. The role of ruthenium in improving the kinetics of hydrogen oxidation and evolution reactions of platinum. Nat. Catal. 2021, 4, 711–718.
Han, L. L.; Ou, P. F.; Liu, W.; Wang, X.; Wang, H. T.; Zhang, R.; Pao, C. W.; Liu, X. J.; Pong, W. F.; Song, J. et al. Design of Ru-Ni diatomic sites for efficient alkaline hydrogen oxidation. Sci. Adv. 2022, 8, eabm3779.
Zhu, Y. P.; Kuo, T. R.; Li, Y. H.; Qi, M. Y.; Chen, G.; Wang, J. L.; Xu, Y. J.; Chen, H. M. Emerging dynamic structure of electrocatalysts unveiled by in situ X-ray diffraction/absorption spectroscopy. Energy Environ. Sci. 2021, 14, 1928–1958.
Li, Q. H.; Peng, H. Q.; Wang, Y. M.; Xiao, L.; Lu, J. T.; Zhuang, L. The comparability of Pt to Pt-Ru in catalyzing the hydrogen oxidation reaction for alkaline polymer electrolyte fuel cells operated at 80 °C. Angew. Chem., Int. Ed. 2019, 58, 1442–1446.
Hamo, E. R.; Singh, R. K.; Douglin, J. C.; Chen, S. A.; Hassine, M. B.; Carbo-Argibay, E.; Lu, S. F.; Wang, H. N.; Ferreira, P. J.; Rosen, B. A. et al. Carbide-supported PtRu catalysts for hydrogen oxidation reaction in alkaline electrolyte. ACS Catal. 2021, 11, 932–947.
Acknowledgements
We gratefully acknowledge the support of this research by the National Natural Science Foundation of China (Nos. 22179034 and 22279030), and the Natural Science Foundation of Heilongjiang Province (No. ZD2023B002).
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Zhang, X., Xie, Y. & Wang, L. Progress and prospect of Pt-based catalysts for electrocatalytic hydrogen oxidation reactions. Nano Res. 17, 960–981 (2024). https://doi.org/10.1007/s12274-023-5987-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-023-5987-1