Abstract
Glial cell line-derived neurotrophic factor (GDNF), a disease-modifying drug for Parkinson’s disease (PD) is in Phase 2 clinical trials (EudraCT number: 2011-003866-34), however it is administered by direct intrastriatal delivery via stereotaxy, which is accompanied with intracranial infection, brain tissue damage, and other complications. In addition, because of complex administration routes, clinical trials of GDNF have yielded contrary results, largely due to differences in dose and concentration brought by intracranial device. Herein, a small molecular agonist SC79 was screened to open blood-brain barrier (BBB) and promote GDNF liposomes to get into brain. SC79 reversibly reduces the expression of claudin-5, one of dominant tight junctions of BBB. Animal study showed SC79 promoted liposomes to enter into brain parenchyma 2.43 times more than that of the control. Motor deficits of PD mice receiving SC79 and brain-targeted GDNF liposomes were recovered by 36.70% and tyrosine hydroxylase positive neurons in striatum were restored by 39.90%. Our combination therapy effectively avoids the side effects such as secondary infection and uneven delivery caused by intracranial injection, improving patients’ compliance and providing valuable research ideas for the clinic.

Similar content being viewed by others
Change history
14 March 2023
An Erratum to this paper has been published: https://doi.org/10.1007/s12274-023-5628-8
References
Bloem, B. R.; Okun, M. S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303.
Kordower, J. H.; Emborg, M. E.; Bloch, J.; Ma, S. Y.; Chu, Y. P.; Leventhal, L.; McBride, J.; Chen, E. Y.; Palfi, S.; Roitberg, B. Z. et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science 2000, 290, 767–773.
Gill, S. S.; Patel, N. K.; Hotton, G. R.; O’Sullivan, K.; McCarter, R.; Bunnage, M.; Brooks, D. J.; Svendsen, C. N.; Heywood, P. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003, 9, 589–595.
Love, S.; Plaha, P.; Patel, N. K.; Hotton, G. R.; Brooks, D. J.; Gill, S. S. Glial cell line-derived neurotrophic factor induces neuronal sprouting in human brain. Nat. Med. 2005, 11, 703–704.
Patel, N. K.; Pavese, N.; Javed, S.; Hotton, G. R.; Brooks, D. J.; Gill, S. S. Benefits of putaminal GDNF infusion in Parkinson disease are maintained after GDNF cessation. Neurology 2013, 81, 1176–1178.
Patel, N. K.; Bunnage, M.; Plaha, P.; Svendsen, C. N.; Heywood, P.; Gill, S. S. Intraputamenal infusion of glial cell line-derived neurotrophic factor in PD: A two-year outcome study. Ann. Neurol. 2005, 57, 298–302.
Gantner, C. W.; de Luzy, I. R.; Kauhausen, J. A.; Moriarty, N.; Niclis, J. C.; Bye, C. R.; Penna, V.; Hunt, C. P. J.; Ermine, C. M.; Pouton, C. W. et al. Viral delivery of GDNF promotes functional integration of human stem cell grafts in Parkinson’s disease. Cell Stem Cell 2020, 26, 511–526.E5.
Whone, A.; Luz, M.; Boca, M.; Woolley, M.; Mooney, L.; Dharia, S.; Broadfoot, J.; Cronin, D.; Schroers, C.; Barua, N. U. et al. Randomized trial of intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease. Brain 2019, 142, 512–525.
Cothros, N.; Medina, A.; Bruno, V. Intermittent intraputamenal glial cell line-derived neurotrophic factor in Parkinson’s disease: Seeking the path to neurorestoration. Mov. Disord. Clin. Pract. 2019, 6, 280–281.
Quinn, T. J.; Drozdowska, B. A. Stroke prediction and the future of prognosis research. Nat. Rev. Neurol. 2019, 15, 311–312.
Gash, D. M.; Gerhardt, G. A.; Bradley, L. H.; Wagner, R.; Slevin, J. T. GDNF clinical trials for Parkinson’s disease: A critical human dimension. Cell Tissue Res. 2020, 382, 65–70.
Abrahao, A.; Meng, Y.; Llinas, M.; Huang, Y. X.; Hamani, C.; Mainprize, T.; Aubert, I.; Heyn, C.; Black, S. E.; Hynynen, K. et al. First-in-human trial of blood-brain barrier opening in amyotrophic lateral sclerosis using MR-guided focused ultrasound. Nat. Commun. 2019, 10, 4373.
Chu, C. Y.; Jablonska, A.; Lesniak, W. G.; Thomas, A. M.; Lan, X. Y.; Linville, R. M.; Li, S.; Searson, P. C.; Liu, G. S.; Pearl, M. et al. Optimization of osmotic blood-brain barrier opening to enable intravital microscopy studies on drug delivery in mouse cortex. J. Control. Release 2020, 317, 312–321.
Zhang, C.; Feng, W.; Li, Y. S.; Kurths, J.; Yu, T. T.; Semyachkina-Glushkovskaya, O.; Zhu, D. Age differences in photodynamic therapy-mediated opening of the blood-brain barrier through the optical clearing skull window in mice. Lasers Surg. Med. 2019, 51, 625–633.
Mathews, M. S.; Shih, E. C.; Zamora, G.; Sun, C. H.; Hirschberg, H.; Blickenstaff, J.; Vo, V.; Madsen, S. J. Photochemical internalization of bleomycin for glioma treatment. J. Biomed. Opt. 2012, 17, 058001.
Tan, L. W.; Wang, Y. Y.; Jiang, Y.; Wang, R.; Zu, J. Z.; Tan, R. Hydroxysafflor yellow a together with blood-brain barrier regulator lexiscan for cerebral ischemia reperfusion injury treatment. ACS Omega 2020, 5, 19151–19164.
Al-Ahmad, A. J.; Pervaiz, I.; Karamyan, V. T. Neurolysin substrates bradykinin, neurotensin and substance P enhance brain microvascular permeability in a human in vitro model. J. Neuroendocrinol. 2021, 33, e12931.
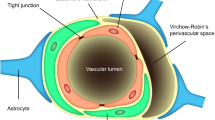
Obermeier, B.; Daneman, R.; Ransohoff, R. M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584–1596.
Wen, L. J.; Wang, K.; Zhang, F. T.; Tan, Y. N.; Shang, X. W.; Zhu, Y.; Zhou, X. Q.; Yuan, H.; Hu, F. Q. AKT activation by SC79 to transiently re-open pathological blood brain barrier for improved functionalized nanoparticles therapy of glioblastoma. Biomaterials 2020, 237, 119793.
Morad, G.; Carman, C. V.; Hagedorn, E. J.; Perlin, J. R.; Zon, L. I.; Mustafaoglu, N.; Park, T. E.; Ingber, D. E.; Daisy, C. C.; Moses, M. A. Tumor-derived extracellular vesicles breach the intact blood-brain barrier via transcytosis. ACS Nano 2019, 13, 13853–13865.
Zhou, X.; Miao, Y. Q.; Wang, Y.; He, S. F.; Guo, L. M.; Mao, J. S.; Chen, M. S.; Yang, Y. T.; Zhang, X. X.; Gan, Y. Tumour-derived extracellular vesicle membrane hybrid lipid nanovesicles enhance siRNA delivery by tumour-homing and intracellular freeway transportation. J. Extracell. Vesicles 2022, 11, e12198.
Munji, R. N.; Soung, A. L.; Weiner, G. A.; Sohet, F.; Semple, B. D.; Trivedi, A.; Gimlin, K.; Kotoda, M.; Korai, M.; Aydin, S. et al. Profiling the mouse brain endothelial transcriptome in health and disease models reveals a core blood-brain barrier dysfunction module. Nat. Neurosci. 2019, 22, 1892–1902.
Sweeney, M. D.; Ayyadurai, S.; Zlokovic, B. V. Pericytes of the neurovascular unit: Key functions and signaling pathways. Nat. Neurosci. 2016, 19, 771–783.
Nutt, J. G.; Burchiel, K. J.; Comella, C. L.; Jankovic, J.; Lang, A. E.; Laws, E. R.; Lozano, A. M.; Penn, R. D.; Simpson, R. K.; Stacy, M. et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology 2003, 60, 69–73.
Slevin, J. T.; Gerhardt, G. A.; Smith, C. D.; Gash, D. M.; Kryscio, R.; Young, B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005, 102, 216–222.
Lang, A. E.; Gill, S.; Patel, N. K.; Lozano, A.; Nutt, J. G.; Penn, R.; Brooks, D. J.; Hotton, G.; Moro, E.; Heywood, P. et al. Randomized controlled trial of intraputamenal glial cell line-derived neurotrophic factor infusion in Parkinson disease. Ann. Neurol. 2006, 59, 459–466.
Bartus, R. T.; Kordower, J. H.; Johnson, E. M. Jr.; Brown, L.; Kruegel, B. R.; Chu, Y.; Baumann, T. L.; Lang, A. E.; Olanow, C. W.; Herzog, C. D. Post-mortem assessment of the short and long-term effects of the trophic factor neurturin in patients with α-synucleinopathies. Neurobiol. Dis. 2015, 78, 162–171.
Barker, R. A.; Björklund, A.; Gash, D. M.; Whone, A.; van Laar, A.; Kordower, J. H.; Bankiewicz, K.; Kieburtz, K.; Saarma, M.; Booms, S. et al. GDNF and Parkinson’s disease: Where next? A summary from a recent workshop. J. Parkinsons. Dis. 2020, 10, 875–891.
Zhu, J. L.; Wu, Y. Y.; Wu, D.; Luo, W. F.; Zhang, Z. Q.; Liu, C. F. SC79, a novel Akt activator, protects dopaminergic neuronal cells from MPP+ and rotenone. Mol. Cell. Biochem. 2019, 461, 81–89.
Lv, J. J.; Hu, W.; Yang, Z.; Tian, L.; Jiang, S.; Ma, Z. Q.; Chen, F. L.; Yang, Y. Focusing on claudin-5: A promising candidate in the regulation of BBB to treat ischemic stroke. Prog. Neurobiol. 2018, 161, 79–96.
Meng, Y.; Pople, C. B.; Lea-Banks, H.; Abrahao, A.; Davidson, B.; Suppiah, S.; Vecchio, L. M.; Samuel, N.; Mahmud, F.; Hynynen, K. et al. Safety and efficacy of focused ultrasound induced blood-brain barrier opening, an integrative review of animal and human studies. J. Control. Release 2019, 309, 25–36.
Acknowledgements
We thank Jingyao Chen and Qiong Huang from the public technology platform of Zhejiang University School of Medicine for their help in immunohistochemical experiments on brain slices. We thank Hangjun Wu in the Center of Cryo-Electron Microscopy (CCEM), Zhejiang University for their technical assistance on two photon imaging. This work was supported by the National Natural Science Foundation of China (No. 81973267) and Natural Science Foundation of Zhejiang Province (No. LD19H300001).
Author information
Authors and Affiliations
Corresponding author
Electronic Supplementary Material
12274_2022_4857_MOESM1_ESM.pdf
SC79 promotes efficient entry of GDNF liposomes into brain parenchyma to repair dopamine neurons through reversible regulation of tight junction proteins
Rights and permissions
About this article
Cite this article
Wu, X., Wang, L., Wang, K. et al. SC79 promotes efficient entry of GDNF liposomes into brain parenchyma to repair dopamine neurons through reversible regulation of tight junction proteins. Nano Res. 16, 2695–2705 (2023). https://doi.org/10.1007/s12274-022-4857-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4857-6