Abstract
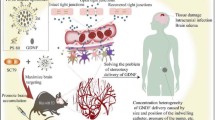
A patient-friendly and efficient treatment method for patients with spinocerebellar ataxia type 3 (SCA3) was provided through a nose-to-brain liposomal system. Initially, PGK1 was overexpressed in HEK 293-84Q-GFP diseased cells (HEK 293-84Q-GFP-PGK1 cells) to confirm its effect on the diseased protein polyQ. A decrease in polyQ expression was demonstrated in HEK 293-84Q-GFP-PGK1 cells compared to HEK 293-84Q-GFP parental cells. Subsequently, PGK1 was encapsulated in a liposomal system to evaluate its therapeutic efficiency in SCA3. The optimized liposomes exhibited a significantly enhanced positive charge, facilitating efficient intracellular protein delivery to the cells. The proteins were encapsulated within the liposomes using an optimized method involving a combination of heat shock and sonication. The liposomal system was further demonstrated to be deliverable to the brain via intranasal administration. PGK1/liposomes were intranasally delivered to SCA3 mice, which subsequently exhibited an amelioration of motor impairment, as assessed via the accelerated rotarod test. Additionally, fewer shrunken morphology Purkinje cells and a reduction in polyQ expression were observed in SCA3 mice that received PGK1/liposomes but not in the untreated, liposome-only, or PGK1-only groups. This study provides a non-invasive route for protein delivery and greater delivery efficiency via the liposomal system for treating neurodegenerative diseases.
Graphical abstract









Similar content being viewed by others
Availability of data and materials
The raw/processed data required to reproduce these findings cannot be shared at this time due to part of an ongoing study.
Abbreviations
- SCA3:
-
Spinocerebellar ataxia type 3
- polyQ:
-
Polyglutamine
- PGK1:
-
Phosphoglycerate kinase 1
- MJD:
-
Machado–Joseph disease
- FDA:
-
Food and Drug Administration
- PET:
-
Positron emission tomography
- FDG:
-
[18F]-2-Fluoro-2-deoxy-D-glucose
- TCA:
-
Tricarboxylic acid
- ALS:
-
Amyotrophic lateral sclerosis (ALS)
- PD:
-
Parkinson’s disease
References
Soong BW, Liu RS. Regional decrease in brain glucose metabolism in asymptomatic gene carriers of Machado-Joseph disease: a preliminary report. Zhonghua Yi Xue Za Zhi (Taipei). 1998;61:121–6.
Soong BW, Liu RS. Positron emission tomography in asymptomatic gene carriers of Machado-Joseph disease. J Neurol Neurosurg Psychiatry. 1998;64:499–504. https://doi.org/10.1136/jnnp.64.4.499.
Taniwaki T, Sakai T, Kobayashi T, Kuwabara Y, Otsuka M, Ichiya Y, Masuda K, Goto I. Positron emission tomography (PET) in Machado-Joseph disease. J Neurol Sci. 1997;145:63–7. https://doi.org/10.1016/s0022-510x(96)00242-0.
Yang ZH, Shi CH, Zhou LN, Li YS, Yang J, Liu YT, Mao CY, Luo HY, Xu GW, Xu YM. Metabolic profiling reveals biochemical pathways and potential biomarkers of spinocerebellar ataxia 3. Front Mol Neurosci. 2019;12:159. https://doi.org/10.3389/fnmol.2019.00159.
Bernstein BE, Hol WG. Crystal structures of substrates and products bound to the phosphoglycerate kinase active site reveal the catalytic mechanism. Biochemistry. 1998;37:4429–36. https://doi.org/10.1021/bi9724117.
Lin CY, Wu CL, Lee KZ, Chen YJ, Zhang PH, Chang CY, Harn HJ, Lin SZ, Tsai HJ. Extracellular PGK1 enhances neurite outgrowth of motoneurons through Nogo66/NgR-independent targeting of NogoA. eLife. 2019;8. https://doi.org/10.7554/eLife.49175.
Chaytow H, Carroll E, Gordon D, Huang YT, van der Hoorn D, Smith HL, Becker T, Becker CG, Faller KME, Talbot K, Gillingwater TH. Targeting phosphoglycerate kinase 1 with terazosin improves motor neuron phenotypes in multiple models of amyotrophic lateral sclerosis. EBioMedicine. 2022;83:104202. https://doi.org/10.1016/j.ebiom.2022.104202.
Lin CY, Tseng HC, Chu YR, Wu CL, Zhang PH, Tsai HJ. Cerebroventricular injection of Pgk1 attenuates MPTP-induced neuronal toxicity in dopaminergic cells in zebrafish brain in a glycolysis-independent manner. Int J Mol Sci. 2022;23. https://doi.org/10.3390/ijms23084150.
Tai W, Zhao P, Gao X. Cytosolic delivery of proteins by cholesterol tagging. Sci Adv. 2020;6:eabb0310. https://doi.org/10.1126/sciadv.abb0310.
Colletier JP, Chaize B, Winterhalter M, Fournier D. Protein encapsulation in liposomes: efficiency depends on interactions between protein and phospholipid bilayer. BMC Biotechnol. 2002;2:9. https://doi.org/10.1186/1472-6750-2-9.
Cheng Q, Wei T, Farbiak L, Johnson LT, Dilliard SA, Siegwart DJ. Selective Organ Targeting (SORT) Nanoparticles for tissue-specific mRNA delivery and CRISPR-Cas gene editing. Nat Nanotechnol. 2020;15:313–20. https://doi.org/10.1038/s41565-020-0669-6.
Thorne RG, Pronk GJ, Padmanabhan V, Frey WH. Delivery of insulin-like growth factor-I to the rat brain and spinal cord along olfactory and trigeminal pathways following intranasal administration. Neuroscience. 2004;127:481–96. https://doi.org/10.1016/j.neuroscience.2004.05.029.
Morgan TM, Soh B. Absolute bioavailability and safety of a novel rivastigmine nasal spray in healthy elderly individuals. Br J Clin Pharmacol. 2017;83:510–6. https://doi.org/10.1111/bcp.13133.
Rajamani K, Liu JW, Wu CH, Chiang IT, You DH, Lin SY, Hsieh DK, Lin SZ, Harn HJ, Chiou TW. n-Butylidenephthalide exhibits protection against neurotoxicity through regulation of tryptophan 2, 3 dioxygenase in spinocerebellar ataxia type 3. Neuropharmacology. 2017;117:434–46. https://doi.org/10.1016/j.neuropharm.2017.02.014.
Liu J, Chang W, Pan L, Liu X, Su L, Zhang W, Li Q, Zheng Y. An improved method of preparing high efficiency transformation escherichia coli with both plasmids and larger DNA fragments. Indian J Microbiol. 2018;58:448–56. https://doi.org/10.1007/s12088-018-0743-z.
Black KA, Priftis D, Perry SL, Yip J, Byun WY, Tirrell M. Protein encapsulation via polypeptide complex coacervation. ACS Macro Lett. 2014;3:1088–91. https://doi.org/10.1021/mz500529v.
Glukhova OE. Liposome drug delivery system across endothelial plasma membrane: role of distance between endothelial cells and blood flow rate. Molecules. 2020;25. https://doi.org/10.3390/molecules25081875.
Erickson HP. Size and shape of protein molecules at the nanometer level determined by sedimentation, gel filtration, and electron microscopy. Biol Proced Online. 2009;11:32–51. https://doi.org/10.1007/s12575-009-9008-x.
Xu X, Khan MA, Burgess DJ. A quality by design (QbD) case study on liposomes containing hydrophilic API: I. Formulation, processing design and risk assessment. Int J Pharm. 2011;419:52–9. https://doi.org/10.1016/j.ijpharm.2011.07.012.
Pavlović N, Mijalković J, Đorđević V, Pecarski D, Bugarski B, Knežević-Jugović Z. Ultrasonication for production of nanoliposomes with encapsulated soy protein concentrate hydrolysate: process optimization, vesicle characteristics and in vitro digestion. Food Chem X. 2022;15:100370. https://doi.org/10.1016/j.fochx.2022.100370.
Meredith ME, Salameh TS, Banks WA. Intranasal delivery of proteins and peptides in the treatment of neurodegenerative diseases. AAPS J. 2015;17:780–7. https://doi.org/10.1208/s12248-015-9719-7.
Borchard G, Lueβen HL, de Boer AG, Verhoef JC, Lehr CM, Junginger HE. The potential of mucoadhesive polymers in enhancing intestinal peptide drug absorption. iii: effects of chitosan-glutamate and carbomer on epithelial tight junctions in vitro. J Control Release. 1996;39:131–8. https://doi.org/10.1016/0168-3659(95)00146-8.
Chen YS, Hong ZX, Lin SZ, Harn HJ. Identifying therapeutic targets for spinocerebellar ataxia type 3/Machado-Joseph disease through integration of pathological biomarkers and therapeutic strategies. Int J Mol Sci. 2020;21:3063. https://doi.org/10.3390/ijms21093063.
Lin YS, Cheng WL, Chang JC, Lin TT, Chao YC, Liu CS. IGF-1 as a potential therapy for spinocerebellar ataxia type 3. Biomedicines. 2022;10. https://doi.org/10.3390/biomedicines10020505.
Tan S, Wang RH, Niu HX, Shi CH, Mao CY, Zhang R, Song B, Sun SL, Liu XJ, Hou HM, Liu YT, Gao Y, Fang H, Kong XD, Xu YM. Nerve growth factor for the treatment of spinocerebellar ataxia type 3: an open-label study. Chin Med J (Engl.). 2015;128:291–4. https://doi.org/10.4103/0366-6999.150087.
Saute JA, da Silva AC, Muller AP, Hansel G, de Mello AS, Maeda F, Vedolin L, Saraiva-Pereira ML, Souza DO, Arpa J, Torres-Aleman I, Portela LV, Jardim LB. Serum insulin-like system alterations in patients with spinocerebellar ataxia type 3. Mov Disord. 2011;26:731–5. https://doi.org/10.1002/mds.23428.
Kieling C, Prestes PR, Saraiva-Pereira ML, Jardim LB. Survival estimates for patients with Machado-Joseph disease (SCA3). Clin Genet. 2007;72:543–5. https://doi.org/10.1111/j.1399-0004.2007.00910.x.
Acknowledgements
We thank the Electron Microscopy Laboratory of Tzu Chi University for their assistance with the TEM support.
Funding
This work was supported by the Ministry of Science and Technology, Taiwan, R.O.C. (MOST-111-2221-E-303-001); the National Science and Technology Council, Taiwan, R.O.C. (NSTC-112-2221-E-303-002); and Hualien Tzu Chi hospital, Buddhist Tzu Chi Medical Foundation, 509 Taiwan, Republic of China (IMAR-110-01-18).
Author information
Authors and Affiliations
Contributions
Y-SC designed the experiments, and Y-SC, Z-XH, Y-TL, E-CT, P-YC, and C-AL performed the experiments. Y-SC, Z-XH, Y-TL, E-CT, P-YC, and C-AL contributed to data compilation and paper preparation. Y-SC, Z-XH, Y-TL, E-CT, P-YC, C-AL, H-JH, T-WC, and S-ZL provided critical feedback on the study and contributed to the preparation of the paper. Y-SC, H-JH, T-WC, and S-ZL provided a resource of papers. Y-SC and S-ZL provided project management for papers. All authors read and approved the final manuscript.
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, YS., Hong, ZX., Lin, YT. et al. Efficiency of PGK1 proteins delivered to the brain via a liposomal system through intranasal route administration for the treatment of spinocerebellar ataxia type 3. Drug Deliv. and Transl. Res. (2023). https://doi.org/10.1007/s13346-023-01498-2
Accepted:
Published:
DOI: https://doi.org/10.1007/s13346-023-01498-2