Abstract
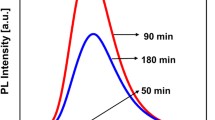
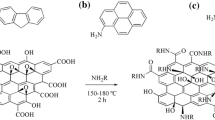
Graphene quantum dots (GQDs) have attracted increasing attention due to their favorable optical properties and have been widely used, e.g., in the biomedical field. However, the properties related to the chemical structure of GQDs, resulting in solvent-dependent optical properties, still remain unclear. Herein, we present the synthesis of long-wavelength emitting GQDs with a size of about 3.6 nm via a solvothermal method using oxo-functionalized graphene (oxo-G) and p-phenylenediamine as precursors and their structural and surface chemical analysis by transmission electron and atomic force microscopy (TEM; AFM) as well as Fourier-transform infrared, Raman, and X-ray photoelectron spectroscopy (FTIR; Raman; XPS). Subsequently, the influence of solvent polarity and proticity on the optical properties of the as-prepared GQDs bearing −OH, −NH2, −COOH and pyridine surface groups was investigated. Based on the results of the absorption and fluorescence (FL) studies, a possible luminescence mechanism is proposed. The observed solvent-induced changes in the spectral position of the FL maximum, FL quantum yield, and FL decay kinetics in protic and aprotic solvents of low and high polarity are ascribed to a combination of polarity effects, intramolecular charge transfer (ICT) processes, and hydrogen bonding. Moreover, the potential of GQDs for the optical sensing of trace amount of water was assessed. The results of our systematic spectroscopic study will promote the rational design of GQDs and shed more light on the FL mechanism of carbon-based fluorescent nanomaterials.

Similar content being viewed by others
References
Li, M. X.; Chen, T.; Gooding, J. J.; Liu, J. Q. Review of carbon and graphene quantum dots for sensing. ACS Sens. 2019, 4, 1732–1748.
Yan, Y. B.; Gong, J.; Chen, J.; Zeng, Z. P.; Huang, W.; Pu, K. Y.; Liu, J. Y.; Chen, P. Recent advances on graphene quantum dots: From chemistry and physics to applications. Adv. Mater. 2019, 31, 1808283.
Chung, S.; Revia, R. A.; Zhang, M. Q. Graphene quantum dots and their applications in bioimaging, biosensing, and therapy. Adv. Mater. 2021, 33, 1904362.
Hadad, C.; González-Domínguez, J. M.; Armelloni, S.; Mattinzoli, D.; Ikehata, M.; Istif, A.; Ostric, A.; Cellesi, F.; Alfieri, C. M.; Messa, P. et al. Graphene quantum dots: From efficient preparation to safe renal excretion. Nano Res. 2021, 14, 674–683.
Mansuriya, B. D.; Altintas, Z. Applications of graphene quantum dots in biomedical sensors. Sensors 2020, 20, 1072.
Liu, W. W.; Li, M.; Jiang, G. P.; Li, G. R.; Zhu, J. B.; Xiao, M. L.; Zhu, Y. F.; Gao, R.; Yu, A. P.; Feng, M. et al. Graphene quantum dots-based advanced electrode materials: Design, synthesis and their applications in electrochemical energy storage and electrocatalysis. Adv. Energy Mater. 2020, 10, 2001275.
Lu, H. T.; Li, W. J.; Dong, H. F.; Wei, M. L. Graphene quantum dots for optical bioimaging. Small 2019, 15, 1902136.
Sousa, H. B. A.; Martins, C. S. M.; Prior, J. A. V. You don’t learn that in school: An updated practical guide to carbon quantum dots. Nanomaterials 2021, 11, 611.
Pan, D. Y.; Zhang, J. C.; Li, Z.; Wu, M. H. Hydrothermal route for cutting graphene sheets into blue-luminescent graphene quantum dots. Adv. Mater. 2010, 22, 734–738.
Wang, S. J.; Cole, I. S.; Zhao, D. Y.; Li, Q. The dual roles of functional groups in the photoluminescence of graphene quantum dots. Nanoscale 2016, 8, 7449–7458.
Shangguan, J. F.; He, D. G.; He, X. X.; Wang, K. M.; Xu, F. Z.; Liu, J. Q.; Tang, J. L.; Yang, X.; Huang, J. Label-free carbon-dots-based ratiometric fluorescence pH nanoprobes for intracellular pH sensing. Anal. Chem. 2016, 88, 7837–7843.
Buncel, E.; Rajagopal, S. Solvatochromism and solvent polarity scales. Acc. Chem. Res. 1990, 23, 226–231.
Marini, A.; Muñoz-Losa, A.; Biancardi, A.; Mennucci, B. What is solvatochromism? J. Phys. Chem. B 2010, 114, 17128–17135.
Reichardt, C. Solvatochromic dyes as solvent polarity indicators. Chem. Rev. 1994, 94, 2319–2358.
Zhu, S. J.; Zhang, J. H.; Qiao, C. Y.; Tang, S. J.; Li, Y. F.; Yuan, W. J.; Li, B.; Tian, L.; Liu, F.; Hu, R. et al. Strongly green-photoluminescent graphenequantum dots for bioimaging applications. Chem. Commun. 2011, 47, 6858–6860.
Niu, X. H.; Li, Y. H.; Shu, H. B.; Wang, J. L. Revealing the underlying absorption and emission mechanism of nitrogen doped graphene quantum dots. Nanoscale 2016, 8, 19376–19382.
Sheely, A.; Gifford, B.; Tretiak, S.; Bishop, A. Tunable optical features of graphene quantum dots from edge functionalization. J. Phys. Chem. C 2021, 125, 9244–9252.
De Arquer, F. P. G.; Talapin, D. V.; Klimov, V. I.; Arakawa, Y.; Bayer, M.; Sargent, E. H. Semiconductor quantum dots: Technological progress and future challenges. Science 2021, 373, eaaz8541.
Lingam, K.; Podila, R.; Qian, H. J.; Serkiz, S.; Rao, A. M. Evidence for edge-state photoluminescence in graphene quantum dots. Adv. Funct. Mater. 2013, 23, 5062–5065.
Yan, Y. B.; Chen, J.; Li, N.; Tian, J. Q.; Li, K. X.; Jiang, J. Z.; Liu, J. Y.; Tian, Q. H.; Chen, P. Systematic bandgap engineering of graphene quantum dots and applications for photocatalytic water splitting and CO2 reduction. ACS Nano 2018, 12, 3523–3532.
Zhu, S. J.; Zhang, J. H.; Tang, S. J.; Qiao, C. Y.; Wang, L.; Wang, H. Y.; Liu, X.; Li, B.; Li, Y. F.; Yu, W. L. et al. Surface chemistry routes to modulate the photoluminescence of graphene quantum dots: From fluorescence mechanism to up-conversion bioimaging applications. Adv. Funct. Mater. 2012, 22, 4732–4740.
Son, D. I.; Kwon, B. W.; Park, D. H.; Seo, W. S.; Yi, Y.; Angadi, B.; Lee, C. L.; Choi, W. K. Emissive ZnO-graphene quantum dots for white-light-emitting diodes. Nat. Nanotechnol. 2012, 7, 465–471.
Yan, Y. X.; Manickam, S.; Lester, E.; Wu, T.; Pang, C. H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519.
Wang, H.; Sun, C.; Chen, X. R.; Zhang, Y.; Colvin, V. L.; Rice, Q.; Seo, J.; Feng, S. Y.; Wang, S. N.; Yu, W. W. Excitation wavelength independent visible color emission of carbon dots. Nanoscale 2017, 9, 1909–1915.
Das, R.; Parveen, S.; Bora, A.; Giri, P. K. Origin of high photoluminescence yield and high sers sensitivity of nitrogen-doped graphene quantum dots. Carbon 2020, 160, 273–286.
Kaniyankandy, S.; Achary, S. N.; Rawalekar, S.; Ghosh, H. N. Ultrafast relaxation dynamics in graphene oxide: Evidence of electron trapping. J. Phys. Chem. C 2011, 115, 19110–19116.
Rajender, G.; Giri, P. K. Formation mechanism of graphene quantum dots and their edge state conversion probed by photoluminescence and Raman spectroscopy. J. Mater. Chem. C 2016, 4, 10852–10865.
Zhang, T. X.; Zhu, J. Y.; Zhai, Y.; Wang, H.; Bai, X. B.; Dong, B.; Wang, H. Y.; Song, H. W. A novel mechanism for red emission carbon dots: Hydrogen bond dominated molecular states emission. Nanoscale 2017, 9, 13042–13051.
Zhu, G. Y.; Zhu, X.; Fan, Q.; Wan, X. L. Raman spectra of amino acids and their aqueous solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2011, 78, 1187–1195.
Chiu, P. L.; Mastrogiovanni, D. D. T.; Wei, D. G.; Louis, C.; Jeong, M.; Yu, G.; Saad, P.; Flach, C. R.; Mendelsohn, R.; Garfunkel, E. et al. Microwave- and nitronium ion-enabled rapid and direct production of highly conductive low-oxygen graphene. J. Am. Chem. Soc. 2012, 134, 5850–5856.
Liu, Q.; Guo, B. D.; Rao, Z. Y.; Zhang, B. H.; Gong, J. R. Strong two-photon-induced fluorescence from photostable, biocompatible nitrogen-doped graphene quantum dots for cellular and deep-tissue imaging. Nano Lett. 2013, 13, 2436–2441.
Zhu, S. J.; Song, Y. B.; Zhao, X. H.; Shao, J. R.; Zhang, J. H.; Yang, B. The photoluminescence mechanism in carbon dots (graphene quantum dots, carbon nanodots, and polymer dots): Current state and future perspective. Nano Res. 2015, 8, 355–381.
Ding, H.; Yu, S. B.; Wei, J. S.; Xiong, H. M. Full-color light-emitting carbon dots with a surface-state-controlled luminescence mechanism. ACS Nano 2016, 10, 484–491.
Dong, Y. Q.; Pang, H. C.; Yang, H. B.; Guo, C. X.; Shao, J. W.; Chi, Y. W.; Li, C. M.; Yu, T. Carbon-based dots co-doped with nitrogen and sulfur for high quantum yield and excitation-independent emission. Angew. Chem., Int. Ed. 2013, 52, 7800–7804.
Ghosh, R.; Mora, A. K.; Nath, S. Disentangling time scales of vibrational cooling, solvation, and hydrogen bond reorganization dynamics using ultrafast transient infrared spectroscopy of formylperylene. J. Phys. Chem. B 2019, 123, 4408–4414.
Ren, T. B.; Xu, W.; Zhang, Q. L.; Zhang, X. X.; Wen, S. Y.; Yi, H. B.; Yuan, L.; Zhang, X. B. Enhancing the anti-solvatochromic two-photon fluorescence for cirrhosis imaging by forming a hydrogen-bond network. Angew. Chem. 2018, 130, 7595–7599.
Liu, H. F.; Yang, J.; Li, Z. H.; Xiao, L. H.; Aryee, A. A.; Sun, Y. Q.; Yang, R.; Meng, H. M.; Qu, L. B.; Lin, Y. H. et al. Hydrogen-bond-induced emission of carbon dots for wash-free nucleus imaging. Anal. Chem. 2019, 91, 9259–9265.
Meierhofer, F.; Dissinger, F.; Weigert, F.; Jungclaus, J.; Müller-Caspary, K.; Waldvogel, S. R.; Resch-Genger, U.; Voss, T. Citric acid based carbon dots with amine type stabilizers: pH-specific luminescence and quantum yield characteristics. J. Phys. Chem. C 2020, 124, 8894–8904.
Liu, H. F.; Sun, Y. Q.; Li, Z. H.; Yang, R.; Yang, J.; Aryee, A. A.; Zhang, X. G.; Ge, J.; Qu, L. B.; Lin, Y. H. Scifinder-guided rational design of fluorescent carbon dots for ratiometric monitoring intracellular pH fluctuations under heat shock. Chin. Chem. Lett. 2019, 30, 1647–1651.
Siraj, N.; El-Zahab, B.; Hamdan, S.; Karam, T. E.; Haber, L. H.; Li, M.; Fakayode, S. O.; Das, S.; Valle, B.; Strongin, R. M. et al. Fluorescence, phosphorescence, and chemiluminescence. Anal. Chem. 2016, 88, 170–202.
Ehtesabi, H.; Hallaji, Z.; Najafi Nobar, S.; Bagheri, Z. Carbon dots with pH-responsive fluorescence: A review on synthesis and cell biological applications. Microchim. Acta 2020, 187, 150.
Wu, Z. L.; Gao, M. X.; Wang, T. T.; Wan, X. Y.; Zheng, L. L.; Huang, C. Z. A general quantitative pH sensor developed with dicyandiamide N-doped high quantum yield graphene quantum dots. Nanoscale 2014, 6, 3868–3874.
Kurniawan, D.; Anjali, B. A.; Setiawan, O.; Ostrikov, K. K.; Chung, Y. G.; Chiang, W. H. Microplasma band structure engineering in graphene quantum dots for sensitive and wide-range pH sensing. ACS Appl. Mater. Interfaces 2022, 14, 1670–1683.
Zhu, S. J.; Song, Y. B.; Wang, J.; Wan, H.; Zhang, Y.; Ning, Y.; Yang, B. Photoluminescence mechanism in graphene quantum dots: Quantum confinement effect and surface/edge state. Nano Today 2017, 13, 10–14.
Xia, C. L.; Zhu, S. J.; Feng, T. L.; Yang, M. X.; Yang, B. Evolution and synthesis of carbon dots: From carbon dots to carbonized polymer dots. Adv. Sci. 2019, 6, 1901316.
Han, M.; Zhu, S. J.; Lu, S. Y.; Song, Y. B.; Feng, T. L.; Tao, S. Y.; Liu, J. J.; Yang, B. Recent progress on the photocatalysis of carbon dots: Classification, mechanism and applications. Nano Today 2018, 19, 201–218.
Shi, X. X.; Hu, Y. L.; Meng, H. M.; Yang, J.; Qu, L. B.; Zhang, X. B.; Li, Z. H. Red emissive carbon dots with dual targetability for imaging polarity in living cells. Sens. Actuators B Chem. 2020, 306, 127582.
Senthamizhan, A.; Fragouli, D.; Balusamy, B.; Patil, B.; Palei, M.; Sabella, S.; Uyar, T.; Athanassiou, A. Hydrochromic carbon dots as smart sensors for water sensing in organic solvents. Nanoscale Adv. 2019, 1, 4258–4267.
Qin, Y. J.; Bai, Y. J.; Huang, P. C.; Wu, F. Y. Dual-emission carbon dots for ratiometric fluorescent water sensing, relative humidity sensing, and anticounterfeiting applications. ACS Appl. Nano Mater. 2021, 4, 10674–10681.
Moniruzzaman, M.; Kim, J. N-doped carbon dots with tunable emission for multifaceted application:Solvatochromism, moisture sensing, pH sensing, and solid state multicolor lighting. Sens. Actuators B Chem. 2019, 295, 12–21.
Acknowledgements
This research is supported by the Deutsche Forschungsgemeinschaft project No. 392444269 (DFG, German Research Foundation), the China Scholarship Council (CSC); We would also like to acknowledge the assistance of the Core Facility BioSupraMol supported by the DFG. C. N. and A. T. acknowledge DFG financial support via the research infrastructure grant INST 275/257-1 FUGG (project No. 313713174), funding through ESF Research Groups 2019 FGR 0080 “EST” and 2020 FGR 0051 “GraphSens” as well as BMWi project ZF4817401VS9 “TDraCon”. Z. H. from Soochow University is acknowledged for conducting TEM measurements. U. R. G and L. S. gratefully acknowledge financial support by the European Metrology Programme for Innovation and Research (EMPIR) as part of the projects 18HLT02 “AeroTox”. The EMPIR initiative is co-funded by the European Union’s Horizon 2020 research and innovation programme and by the EMPIR participating states.
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4752_MOESM1_ESM.pdf
Polarity, intramolecular charge transfer, and hydrogen bond co-mediated solvent effects on the optical properties of graphene quantum dots
Rights and permissions
About this article
Cite this article
Hu, Y., Neumann, C., Scholtz, L. et al. Polarity, intramolecular charge transfer, and hydrogen bond co-mediated solvent effects on the optical properties of graphene quantum dots. Nano Res. 16, 45–52 (2023). https://doi.org/10.1007/s12274-022-4752-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4752-1