Abstract
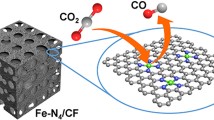
Electroreduction of greenhouse gas CO2 into value-added fuels and chemicals provides a promising pathway to address the issues of energy crisis and environmental change. However, the regulations of the selectivity towards C2 product and the competing hydrogen evolution reaction (HER) are major challenges for CO2 reduction reaction (CO2RR). Here, we develop an interface-enhanced strategy by depositing a thin layer of nitrogen-doped graphene (N-G) on a Cu foam surface (Cu-N-G) to selectively promote the ethanol pathway in CO2RR. Compared to the undetectable ethanol selectivity of pure Cu and Cu@graphene (Cu-G), Cu-N-G has boosted the ethanol selectivity to 33.1% in total Faradic efficiency (FE) at −0.8 V vs. reversible hydrogen electrode (RHE). The experimental and density functional theory (DFT) results verify that the interconnected graphene coating can not only serve as the fast charge transport channel but also provide confined nanospace for mass transfer. The N doping can not only trigger the intrinsic interaction between N in N-G and CO2 molecule for enriching the local concentration of reactants but also promote the average valence state of Cu for C-C coupling pathways. The confinement effect at the interface of Cu-N-G can not only provide high adsorbed hydrogen (Had) coverage but also stabilize the key ⋆HCCHOH intermediate towards ethanol pathway. The provided interface-enhanced strategy herein is expected to inspire the design of Cu-based CO2RR electrocatalysts towards multi-carbon products.

Similar content being viewed by others
References
Wang, X.; Wang, Z. Y.; García de Arquer, F. P.; Dinh, C. T.; Ozden, A.; Li, Y. C.; Nam, D. H.; Li, J.; Liu, Y. S.; Wicks, J. et al. Efficient electrically powered CO2-to-ethanol via suppression of deoxygenation. Nat. Energy 2020, 5, 478–486.
Yu, J. L.; Wang, J.; Ma, Y. B.; Zhou, J. W.; Wang, Y. H.; Lu, P. Y.; Yin, J. W.; Ye, R. Q.; Zhu, Z. L.; Fan, Z. X. Recent progresses in electrochemical carbon dioxide reduction on copper-based catalysts toward multicarbon products. Adv. Funct. Mater. 2021, 31, 2102151.
Jia, Y. F.; Li, F.; Fan, K.; Sun, L. C. Cu-based bimetallic electrocatalysts for CO2 reduction. Adv. Powder Mater. 2022, 1, 100012.
Zhou, Y. S.; Yeo, B. S. Formation of C-C bonds during electrocatalytic CO2 reduction on non-copper electrodes. J. Mater. Chem. A 2020, 8, 23162–23186.
Liang, H. Q.; Zhao, S. Q.; Hu, X. M.; Ceccato, M.; Skrydstrup, T.; Daasbjerg, K. Hydrophobic copper interfaces boost electroreduction of carbon dioxide to ethylene in water. ACS Catal. 2021, 11, 958–966.
Li, F. W.; Li, Y. C.; Wang, Z. Y.; Li, J.; Nam, D. H.; Lum, Y.; Luo, M. C.; Wang, X.; Ozden, A.; Hung, S. F. et al. Cooperative CO2-to-ethanol conversion via enriched intermediates at molecule-metal catalyst interfaces. Nat. Catal. 2020, 3, 75–82.
Zhang, S. L.; Sun, L.; Fan, Q. N.; Zhang, F. L.; Wang, Z. J.; Zou, J. S.; Zhao, S. Y.; Mao, J. F.; Guo, Z. P. Challenges and prospects of lithium-CO2 batteries. Nano Res. Energy 2022, 1, e9120001.
Yuan, Q.; Yang, H.; Xie, M.; Cheng, T. Theoretical research on the electroreduction of carbon dioxide. Acta Phys. Chim. Sin. 2021, 37, 2010040.
Li, J. J.; Abbas, S. U.; Wang, H. Q.; Zhang, Z. C.; Hu, W. P. Recent advances in interface engineering for electrocatalytic CO2 reduction reaction. Nano-Micro Lett. 2021, 13, 216.
Yang, C. H.; Li, S. Y.; Zhang, Z. C.; Wang, H. Q.; Liu, H. L.; Jiao, F.; Guo, Z. G.; Zhang, X. T.; Hu, W. P. Organic-inorganic hybrid nanomaterials for electrocatalytic CO2 reduction. Small 2020, 16, 2001847.
Garza, A. J.; Bell, A. T.; Head-Gordon, M. Mechanism of CO2 reduction at copper surfaces: Pathways to C2 products. ACS Catal. 2018, 8, 1490–1499.
Wang, N.; Miao, R. K.; Lee, G.; Vomiero, A.; Sinton, D.; Ip, A. H.; Liang, H. Y.; Sargent, E. H. Suppressing the liquid product crossover in electrochemical CO2 reduction. SmartMat 2021, 2, 12–16.
Zhou, Y. J.; Liang, Y. Q.; Fu, J. W.; Liu, K.; Chen, Q.; Wang, X. Q.; Li, H. M.; Zhu, L.; Hu, J. H.; Pan, H. et al. Vertical cu nanoneedle arrays enhance the local electric field promoting C2 hydrocarbons in the CO2 electroreduction. Nano Lett. 2022, 22, 1963–1970.
Choi, C.; Kwon, S.; Cheng, T.; Xu, M. J.; Tieu, P.; Lee, C.; Cai, J.; Lee, H. M.; Pan, X. Q.; Duan, X. F. et al. Highly active and stable stepped Cu surface for enhanced electrochemical CO2 reduction to C2H4. Nat. Catal. 2020, 3, 804–812.
Han, L.; Tian, B. Q.; Gao, X. X.; Zhong, Y.; Wang, S. N.; Song, S. C.; Wang, Z. L.; Zhang, Y.; Kuang, Y.; Sun, X. M. Copper nanowire with enriched high-index facets for highly selective CO2 reduction. SmartMat 2022, 3, 142–150.
Zhang, H.; He, C. H.; Han, S. M.; Du, Z. Y.; Wang, L.; Yun, Q. B.; Cao, W. B.; Zhang, B. W.; Tian, Y. H.; Lu, Q. P. Crystal facet-dependent electrocatalytic performance of metallic Cu in CO2 reduction reactions. Chin. Chem. Lett. 2022, 33, 3641–3649.
Yang, D. R.; Zuo, S. W.; Yang, H. Z.; Wang, X. Single-unit-cell catalysis of CO2 electroreduction over sub-1 nm Cu9S5 nanowires. Adv. Energy Mater. 2021, 11, 2100272.
Wang, Y. H.; Jiang, W. J.; Yao, W.; Liu, Z. L.; Liu, Z.; Yang, Y.; Gao, L. Z. Advances in electrochemical reduction of carbon dioxide to formate over bismuth-based catalysts. Rare Met. 2021, 40, 2327–2353.
Zhu, Y. T.; Gao, Z. Q.; Zhang, Z. C.; Lin, T.; Zhang, Q. H.; Liu, H. L.; Gu, L.; Hu, W. P. Selectivity regulation of CO2 electroreduction on asymmetric AuAgCu tandem heterostructures. Nano Res., in press, https://doi.org/10.1007/s12274-022-4234-5.
Zhou, Y. S.; Che, F. L.; Liu, M.; Zou, C. Q.; Liang, Z. Q.; De Luna, P.; Yuan, H. F.; Li, J.; Wang, Z. Q.; Xie, H. P. et al. Dopant-induced electron localization drives CO2 reduction to C2 hydrocarbons. Nat. Chem. 2018, 10, 974–980.
Wang, N.; Yao, K. L.; Vomiero, A.; Wang, Y. H.; Liang, H. Y. Inhibiting carbonate formation using CO2-CO-C2+ tandems. SmartMat 2021, 2, 423–425.
Sun, C. Y.; Zhao, Z. W.; Liu, H.; Wang, H. Q. Core—shell nanostructure for supra-photothermal CO2 catalysis. Rare Met. 2022, 41, 1403–1405.
Wang, H. Q. Nanostructure@metal-organic frameworks (MOFs) for catalytic carbon dioxide (CO2) conversion in photocatalysis, electrocatalysis, and thermal catalysis. Nano Res. 2022, 15, 2834–2854.
Jiang, L. W.; Dong, D. J.; Lu, Y. C. Design strategies for low temperature aqueous electrolytes. Nano Res. Energy 2022, 1, e9120003.
Li, Z.; Yang, Y.; Yin, Z. L.; Wei, X.; Peng, H. Q.; Lyu, K. J.; Wei, F. Y.; Xiao, L.; Wang, G. W.; Abruña, H. D. et al. Interface-enhanced catalytic selectivity on the C2 products of CO2 electroreduction. ACS Catal. 2021, 11, 2473–2482.
Wang, Y. C.; Xu, L.; Zhan, L. S.; Yang, P. Y.; Tang, S. H.; Liu, M. J.; Zhao, X.; Xiong, Y.; Chen, Z. Y.; Lei, Y. P. Electron accumulation enables Bi efficient CO2 reduction for formate production to boost clean Zn-CO2 batteries. Nano Energy 2022, 92, 106780.
Zhang, Z.; Yu, L.; Tu, Y. C.; Chen, R. X.; Wu, L. H.; Zhu, J. F.; Deng, D. H. Unveiling the active site of metal-free nitrogen-doped carbon for electrocatalytic carbon dioxide reduction. Cell Rep. Phys. Sci. 2020, 1, 100145.
Gao, Z. Q.; Li, J. J.; Zhang, Z. C.; Hu, W. P. Recent advances in carbon-based materials for electrochemical CO2 reduction reaction. Chin. Chem. Lett. 2022, 33, 2270–2280.
Meng, Y. C.; Kuang, S. Y.; Liu, H.; Fan, Q.; Ma, X. B.; Zhang, S. Recent advances in electrochemical CO2 reduction using copper-based catalysts. Acta Phys. Chim. Sin. 2021, 37, 2006034.
Jin, H. D.; Xiong, L. K.; Zhang, X.; Lian, Y. B.; Chen, S.; Lu, Y. T.; Deng, Z.; Peng, Y. Cu-based catalyst derived from nitrogen-containing metal organic frameworks for electroreduction of CO2. Acta Phys. Chim. Sin. 2021, 37, 2006017.
Yang, D. R.; Zuo, S. W.; Yang, H. Z.; Zhou, Y.; Lu, Q. C.; Wang, X. Tailoring layer number of 2D porphyrin-based MOFs towards photocoupled electroreduction of CO2. Adv. Mater. 2022, 34, 2107293.
Yang, H. Z.; Yang, D. R.; Zhou, Y.; Wang, X. Polyoxometalate interlayered zinc-metallophthalocyanine molecular layer sandwich as photocoupled electrocatalytic CO2 reduction catalyst. J. Am. Chem. Soc. 2021, 143, 13721–13730.
Chen, Z. P.; Ren, W. C.; Gao, L. B.; Liu, B. L.; Pei, S. F.; Cheng, H. M. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 2011, 10, 424–428.
Wang, H. Q.; Zhang, W. J.; Zhang, X. W.; Hu, S. X.; Zhang, Z. C.; Zhou, W. J.; Liu, H. Multi-interface collaboration of graphene cross-linked NiS-NiS2-Ni3S4 polymorph foam towards robust hydrogen evolution in alkaline electrolyte. Nano Res. 2021, 14, 4857–4864.
Zeng, J.; Bi, L. Y.; Cheng, Y. H.; Xu, B. M.; Jen, A. K. Y. Self-assembled monolayer enabling improved buried interfaces in blade-coated perovskite solar cells for high efficiency and stability. Nano Res. Energy 2022, 1, e9120004.
Li, M. R.; Idros, M. N.; Wu, Y. M.; Burdyny, T.; Garg, S.; Zhao, X. S.; Wang, G.; Rufford, T. E. The role of electrode wettability in electrochemical reduction of carbon dioxide. J. Mater. Chem. A 2021, 9, 19369–19409.
Yang, C. H.; Nosheen, F.; Zhang, Z. C. Recent progress in structural modulation of metal nanomaterials for electrocatalytic CO2 reduction. Rare Met. 2021, 40, 1412–1430.
Fang, B.; Xing, Z. P.; Sun, D. D.; Li, Z. Z.; Zhou, W. Hollow semiconductor photocatalysts for solar energy conversion. Adv. Powder Mater. 2022, 1, 100021.
Chen, J. J.; Mao, Z. Y.; Zhang, L. X.; Wang, D. J.; Xu, R.; Bie, L. J.; Fahlman, B. D. Nitrogen-deficient graphitic carbon nitride with enhanced performance for lithium ion battery anodes. ACS Nano 2017, 11, 12650–12657.
Liu, Y. M.; Yang, H. L.; Fan, X. F.; Shan, B.; Meyer, T. J. Promoting electrochemical reduction of CO2 to ethanol by B/N-doped sp3/sp2 nanocarbon electrode. Chin. Chem. Lett. 2022, 33, 4691–4694.
Duan, Y. X.; Meng, F. L.; Liu, K. H.; Yi, S. S.; Li, S. J.; Yan, J. M.; Jiang, Q. Amorphizing of Cu nanoparticles toward highly efficient and robust electrocatalyst for CO2 reduction to liquid fuels with high faradaic efficiencies. Adv. Mater. 2018, 30, 1706194.
Li, D.; Liu, T. T.; Huang, L. L.; Wu, J.; Li, J. N.; Zhen, L.; Feng, Y. J. Selective CO2-to-formate electrochemical conversion with core-shell structured Cu2O/Cu@C composites immobilized on nitrogen-doped graphene sheets. J. Mater. Chem. A 2020, 8, 18302–18309.
Wu, Z. Z.; Zhang, X. L.; Niu, Z. Z.; Gao, F. Y.; Yang, P. P.; Chi, L. P.; Shi, L.; Wei, W. S.; Liu, R.; Chen, Z. et al. Identification of Cu(100)/Cu(111) interfaces as superior active sites for CO dimerization during CO2 electroreduction. J. Am. Chem. Soc. 2022, 144, 259–269.
Zhang, W.; Huang, C. Q.; Xiao, Q.; Yu, L.; Shuai, L.; An, P. F.; Zhang, J.; Qiu, M.; Ren, Z. F.; Yu, Y. Atypical oxygen-bearing copper boosts ethylene selectivity toward electrocatalytic CO2 reduction. J. Am. Chem. Soc. 2020, 142, 11417–11427.
Liu, M. X.; Xu, Y. K.; Meng, Y.; Wang, L. J.; Wang, H.; Huang, Y. C.; Onishi, N.; Wang, L.; Fan, Z. J.; Himeda, Y. Heterogeneous catalysis for carbon dioxide mediated hydrogen storage technology based on formic acid. Adv. Energy Mater., in press, https://doi.org/10.1002/aenm.202200817.
Hao, J. C.; Zhuang, Z. C.; Hao, J. C.; Cao, K. C.; Hu, Y. X.; Wu, W. B.; Lu, S. L.; Wang, C.; Zhang, N.; Wang, D. S. et al. Strain relaxation in metal alloy catalysts steers the product selectivity of electrocatalytic CO2 reduction. ACS Nano 2022, 16, 3251–3263.
Hao, J. C.; Zhuang, Z. C.; Hao, J. C.; Wang, C.; Lu, S. L.; Duan, F.; Xu, F. P.; Du, M. L.; Zhu, H. Interatomic electronegativity offset dictates selectivity when catalyzing the CO2 reduction reaction. Adv. Energy Mater., in press, https://doi.org/10.1002/aenm.202200579.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 21907043 and 21801153) and Shandong Provincial Natural Science Foundation (No. ZR2019BB025).
Author information
Authors and Affiliations
Corresponding authors
Electronic Supplementary Material
12274_2022_4698_MOESM1_ESM.pdf
Confined interface engineering of self-supported Cu@N-doped graphene for electrocatalytic CO2 reduction with enhanced selectivity towards ethanol
Rights and permissions
About this article
Cite this article
Zang, D., Gao, X.J., Li, L. et al. Confined interface engineering of self-supported Cu@N-doped graphene for electrocatalytic CO2 reduction with enhanced selectivity towards ethanol. Nano Res. 15, 8872–8879 (2022). https://doi.org/10.1007/s12274-022-4698-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-022-4698-3