Abstract
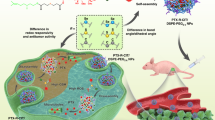
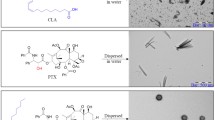
Prodrug-based nanoassembly emerges as a hopeful way for the efficient delivery of antitumor drugs, with carrier-free structure and ultra-high drug loading. Carbon chains are widely used to design self-assembling prodrugs. The impacts of the length of carbon chains on the self-assembly stability, drug delivery efficiency and antitumor effect of prodrugs have not been fully elucidated. Here, three paclitaxel prodrugs were synthesized by conjugating paclitaxel with octanol (C8), decanol (C10) or dodecanol (C12) through disulfide bond. The three prodrugs could form homogeneous nanoparticles, with over 50% drug loading and redox dual-responsivity. Interestingly, the length extension of carbon chains ameliorates the self-assembly and the colloidal stability of prodrugs, thus improving the drug delivery efficiency. The optimal paclitaxel-dodecanol prodrug nanoassemblies exhibit better antitumor efficacy than Taxol and Abraxane. These findings are meaningful for the rational design of advanced nanomedicines in cancer therapy.

Similar content being viewed by others
References
Smith, R. A.; Andrews, K. S.; Brooks, D.; Fedewa, S. A.; Manassaram-Baptiste, D.; Saslow, D.; Wender, R. C. Cancer screening in the United States, 2019: A review of current American Cancer Society guidelines and current issues in cancer screening. CA: Cancer J. Clin. 2019, 69, 184–210.
Kaufmann, S. H.; Earnshaw, W. C. Induction of apoptosis by cancer chemotherapy. Exp. Cell Res. 2000, 256, 42–49.
Chabner, B. A.; Roberts, T. G. Jr. Chemotherapy and the war on cancer. Nat. Rev. Cancer 2005, 5, 65–72.
Rowinsky, E. K.; Donehower, R. C. Paclitaxel (Taxol). N. Engl. J. Med. 1995, 332, 1004–1014.
Sun, B. J.; Luo, C.; Zhang, X. B.; Guo, M. R.; Sun, M. C.; Yu, H.; Chen, Q.; Yang, W. Q.; Wang, M. L.; Zuo, S. Y. et al. Probing the impact of sulfur/selenium/carbon linkages on prodrug nanoassemblies for cancer therapy. Nat. Commun. 2019, 10, 3211.
Sun, B. J.; Luo, C.; Yu, H.; Zhang, X. B.; Chen, Q.; Yang, W. Q.; Wang, M. L.; Kan, Q. M.; Zhang, H. T.; Wang, Y. J. et al. Disulfide bond-driven oxidation- and reduction-responsive prodrug nanoassemblies for cancer therapy. Nano Lett. 2018, 18, 3643–3650.
Li, L. X.; Zuo, S. Y.; Dong, F. D.; Liu, T.; Gao, Y. L.; Yang, Y. X.; Wang, X.; Sun, J.; Sun, B. J; He, Z. G. Small changes in the length of diselenide bond-containing linkages exert great influences on the antitumor activity of docetaxel homodimeric prodrug nanoassemblies. Asian J. Pharm. Sci. 2021, 16, 337–349.
Yang, Y. X.; Sun, B. J.; Zuo, S. Y.; Li, X. M.; Zhou, S.; Li, L. X.; Luo, C.; Liu, H. Z.; Cheng, M. S.; Wang, Y. J. et al. Trisulfide bondmediated doxorubicin dimeric prodrug nanoassemblies with high drug loading, high self-assembly stability, and high tumor selectivity. Sci. Adv. 2020, 6, eabc1725.
Zuo, S. Y.; Sun, B. J.; Yang, Y. X.; Zhou, S.; Zhang, Y.; Guo, M. R.; Sun, M. C.; Luo, C.; He, Z. G.; Sun, J. Probing the superiority of diselenium bond on docetaxel dimeric prodrug nanoassemblies: Small roles taking big responsibilities. Small 2020, 16, 2005039.
Li, S. M.; Shan, X. Z.; Wang, Y. Q.; Chen, Q.; Sun, J.; He, Z. G.; Sun, B. J.; Luo, C. Dimeric prodrug-based nanomedicines for cancer therapy. J. Control. Release 2020, 326, 510–522.
Sharma, A.; Lee, M. G.; Won, M.; Koo, S.; Arambula, J. F.; Sessler, J. L.; Chi, S. G.; Kim, J. S. Targeting heterogeneous tumors using a multifunctional molecular prodrug. J. Am. Chem. Soc. 2019, 141, 15611–15618.
Shi, J. J.; Kantoff, P. W.; Wooster, R.; Farokhzad, O. C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37.
Hartshorn, C. M.; Bradbury, M. S.; Lanza, G. M.; Nel, A. E.; Rao, J. H.; Wang, A. Z.; Wiesner, U. B.; Yang, L.; Grodzinski, P. Nanotechnology strategies to advance outcomes in clinical cancer care. ACS Nano 2018, 12, 24–43.
Peng, Y.; Chen, L.; Ye, S.; Kang, Y.; Liu, J. Q.; Zeng, S.; Yu, L. S. Research and development of drug delivery systems based on drug transporter and nano-formulation. Asian J. Pharm. Sci. 2020, 15, 220–236.
Sun, B. J.; Luo, C.; Cui, W. P.; Sun, J.; He, Z. G. Chemotherapy agent-unsaturated fatty acid prodrugs and prodrug-nanoplatforms for cancer chemotherapy. J. Control. Release 2017, 264, 145–159.
Lv, J.; Wang, C. P.; Li, H. R.; Li, Z.; Fan, Q. Q.; Zhang, Y.; Li, Y. W.; Wang, H.; Cheng, Y. Y. Bifunctional and bioreducible dendrimer bearing a fluoroalkyl tail for efficient protein delivery both in vitro and in vivo. Nano Lett. 2020, 20, 8600–8607.
Li, S. L.; Saw, P. E.; Lin, C. H.; Nie, Y.; Tao, W.; Farokhzad, O. C.; Zhang, L.; Xu, X. D. Redox-responsive polyprodrug nanoparticles for targeted siRNA delivery and synergistic liver cancer therapy. Biomaterials 2020, 234, 119760.
Xiao, X.; Wang, K. W.; Zong, Q. Y.; Tu, Y. L.; Dong, Y. S.; Yuan, Y. Y. Polyprodrug with glutathione depletion and cascade drug activation for multi-drug resistance reversal. Biomaterials 2021, 270, 120649.
Zheng, Y. X.; Lei, J.; Li, Q.; Jin, X.; Li, Q. Y.; Li Y. Interfacial cationization to quicken redox-responsive drug release. Chem. Commun. 2021, 57, 2943–2946.
Xiao, F.; Chen, Z.; Wei, Z. X.; Tian, L. L. Hydrophobic interaction: A promising driving force for the biomedical applications of nucleic acids. Adv. Sci. 2020, 7, 2001048.
Chakraborty, S.; Grego, A. S.; Garai, S.; Baranov, M.; Müller, A.; Weinstock, I. A. Alcohols as latent hydrophobes: Entropically driven uptake of 1,2-diol functionalized ligands by a porous capsule in water. J. Am. Chem. Soc. 2019, 141, 9170–9174.
Allen, C. D.; Link, A. J. Self-assembly of catenanes from lasso peptides. J. Am. Chem. Soc. 2016, 138, 14214–14217.
Cai, K. M.; He, X.; Song, Z. Y.; Yin, Q.; Zhang, Y. F.; Uckun, F. M.; Jiang, C.; Cheng, J. J. Dimeric drug polymeric nanoparticles with exceptionally high drug loading and quantitative loading efficiency. J. Am. Chem. Soc. 2015, 137, 3458–3461.
Demangeat, C.; Dou, Y. X.; Hu, B.; Bretonnière, Y.; Andraud, C.; D’Aléo, A.; Wu, J. W.; Kim, E.; Bahers, T. L.; Attias, A. J. σ-Conjugation and H-bond-directed supramolecular self-assembly: Key features for efficient long-lived room temperature phosphorescent organic molecular crystals. Angew. Chem., Int. Ed. 2021, 60, 2446–2454.
Luo, C.; Sun, J.; Sun B. J.; Liu, D.; Miao, L.; Goodwin, T. J.; Huang, L.; He, Z. G. Facile fabrication of tumor redox-sensitive nanoassemblies of small-molecule oleate prodrug as potent chemotherapeutic nanomedicine. Small 2016, 12, 6353–6362.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (No. 82073777), Liaoning Revitalization Talents Program (No. XTYC180801), China Postdoctoral Innovative Talents Support Program (No. BX20190219), and China Postdoctoral Science Foundation (No. 2019M661134).
Author information
Authors and Affiliations
Corresponding authors
Additional information
Declaration of interest
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Electronic Supplementary Material
12274_2021_3895_MOESM1_ESM.pdf
Minor change in the length of carbon chain has a great influence on the antitumor effect of paclitaxel-fatty alcohol prodrug nanoassemblies: Small roles, big impacts
Rights and permissions
About this article
Cite this article
Wang, X., Li, L., Wang, D. et al. Minor change in the length of carbon chain has a great influence on the antitumor effect of paclitaxel-fatty alcohol prodrug nanoassemblies: Small roles, big impacts. Nano Res. 15, 3367–3375 (2022). https://doi.org/10.1007/s12274-021-3895-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12274-021-3895-9