Abstract
Aim
To evaluate the impact of polymorphisms in the cytochrome P450 (CYP) 2C9, 2C19 and 2C8 genes on the risk of mild hypoglycaemic attacks in patients treated with sulphonylureas.
Methods
One hundred and eight type 2 diabetic patients (50 men, 58 women), treated with oral antidiabetics, including at least one from the sulphonylurea group (glimepiride n = 50, gliclazide n = 46, or glipizide n = 12) for 3 months or longer, were included in the study. Symptoms of hypoglycaemia (sweating, tremor, anxiety and palpitations) during a 3 month period were recorded and confirmed by home glucose measurements. Gender, age, body mass index, creatinine clearance, HbA1c, oral antidiabetic dose and concomitant medication were assessed together with functional CYP2C9, CYP2C19 and CYP2C8 polymorphisms, analysed by real-time PCR methods.
Results
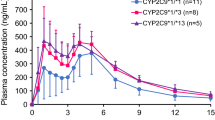
Fifteen patients (eight men, seven women) reported hypoglycaemia symptoms which were validated by their home glucose measurements (< 70 mg/dl). Heterozygosity and homozygosity for CYP2C9 variant alleles (*2 or *3) tended to be more frequent among patients who reported hypoglycaemic attacks (60 and 7%) than those who did not (39 and 3%). Similarly, the CYP2C8*1/*3 genotype tended to be more frequent in patients with (47%) than without (27%) hypoglycaemia, while no such trend was observed for CYP2C19 variants. However, only in the gliclazide group a significant association between CYP2C9 genotype and hypoglycaemic attacks was observed (P = 0.035). None of the other covariates showed any significant association with the risk of hypoglycaemic attacks.
Conclusions
CYP2C9 polymorphisms leading to decreased enzyme activity show a modest impact on the risk of mild hypoglycaemia attacks during oral antidiabetic treatment, with a significant association in patients treated with gliclazide.
Similar content being viewed by others
References
Asplund K, Wiholm BE, Lithner F (1983) Glibenclamide-associated hypoglycaemia: a report on 57 cases. Diabetologia 24(6):412–417
Holstein A, Egberts EH (2003) Risk of hypoglycaemia with oral antidiabetic agents in patients with type 2 diabetes. Exp Clin Endocrinol Diabetes 111(7):405–414
Kirchheiner J, Roots I, Goldammer M, Rosenkranz B et al (2005) Effect of genetic polymorphisms in cytochrome P450 (CYP) 2 C9 and CYP2C8 on the pharmacokinetics of oral antidiabetic drugs: clinical relevance. Clin Pharmacokinet 44(12):1209–1225
Elliot DJ, Suharjono LBC, Gillam EM et al (2007) Identification of the human cytochromes P450 catalysing the rate-limiting pathways of gliclazide elimination. Br J Clin Pharmacol 64(4):450–457
Tan B, Zhang YF, Chen XY, Zhao XH et al (2009) The effects of CYP2C9 and CYP2C19 genetic polymorphisms on the pharmacokinetics and pharmacodynamics of glipizide in Chinese subjects. Eur J Clin Pharmacol 66(2):145–151
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2 C9 polymorphisms: a comprehensive review of the in-vitro and human data. Pharmacogenetics 12(3):251–263
Yasar U, Eliasson E, Dahl ML, Johansson I et al (1999) Validation of methods for CYP2C9 genotyping: frequencies of mutant alleles in a Swedish population. Biochem Biophys Res Commun 254(3):628–631
Ragia G, Petridis I, Tavridou A, Christakidis D et al (2009) Presence of CYP2C9*3 allele increases risk for hypoglycemia in type 2 diabetic patients treated with sulfonylureas. Pharmacogenomics 10(11):1781–1787
Holstein A, Plaschke A, Ptak M, Egberts EH et al (2005) Association between CYP2C9 slow metabolizer genotypes and severe hypoglycaemia on medication with sulphonylurea hypoglycaemic agents. Br J Clin Pharmacol 60(1):103–106
Holstein A, Hammer C, Hahn M, Kulamadayil NS et al (2011) Severe sulfonylurea-induced hypoglycemia: a problem of uncritical prescription and deficiencies of diabetes care in geriatric patients. Expert Opin Drug Saf 9(5):675–681
Becker ML, Visser LE, Trienekens PH, Hofman A et al (2008) Cytochrome P450 2 C9 *2 and *3 polymorphisms and the dose and effect of sulfonylurea in type II diabetes mellitus. Clin Pharmacol Ther 83(2):288–292
Desta Z, Zhao X, Shin JG, Flockhart DA (2002) Clinical significance of the cytochrome P450 2 C19 genetic polymorphism. Clin Pharmacokinet 41(12):913–958
Sim SC, Risinger C, Dahl ML, Aklillu E et al (2006) A common novel CYP2C19 gene variant causes ultrarapid drug metabolism relevant for the drug response to proton pump inhibitors and antidepressants. Clin Pharmacol Ther 79(1):103–113
Ragia G, Arvanitidis KI, Tavridou A, Manolopoulos VG (2009) Need for reassessment of reported CYP2C19 allele frequencies in various populations in view of CYP2C19*17 discovery: the case of Greece. Pharmacogenomics 10(1):43–49
Shao H, Ren XM, Liu NF, Chen GM et al (2010) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics and pharmacodynamics of gliclazide in healthy Chinese Han volunteers. J Clin Pharm Ther 35(3):351–360
Zhang Y, Si D, Chen X, Lin N et al (2007) Influence of CYP2C9 and CYP2C19 genetic polymorphisms on pharmacokinetics of gliclazide MR in Chinese subjects. Br J Clin Pharmacol 64(1):67–74
Dai D, Zeldin DC, Blaisdell JA, Chanas B et al (2001) Polymorphisms in human CYP2C8 decrease metabolism of the anticancer drug paclitaxel and arachidonic acid. Pharmacogenetics 11(7):597–607
Aquilante CL, Bushman LR, Knutsen SD, Burt LE et al (2008) Influence of SLCO1B1 and CYP2C8 gene polymorphisms on rosiglitazone pharmacokinetics in healthy volunteers. Hum Genomics 3(1):7–16
Niemi M, Leathart JB, Neuvonen M, Backman JT et al (2003) Polymorphism in CYP2C8 is associated with reduced plasma concentrations of repaglinide. Clin Pharmacol Ther 74(4):380–387
Tornio A, Niemi M, Neuvonen PJ, Backman JT (2008) Trimethoprim and the CYP2C8*3 allele have opposite effects on the pharmacokinetics of pioglitazone. Drug Metab Dispos 36(1):73–80
Bahadur N, Leathart JB, Mutch E, Steimel-Crespi D et al (2002) CYP2C8 polymorphisms in Caucasians and their relationship with paclitaxel 6alpha-hydroxylase activity in human liver microsomes. Biochem Pharmacol 64(11):1579–1589
Yasar U, Lundgren S, Eliasson E, Bennet A et al (2002) Linkage between the CYP2C8 and CYP2C9 genetic polymorphisms. Biochem Biophys Res Commun 299(1):25–28
Xu H, Williams KM, Liauw WS, Murray M et al (2008) Effects of St John's wort and CYP2C9 genotype on the pharmacokinetics and pharmacodynamics of gliclazide. Br J Pharmacol 153(7):1579–1586
Wang R, Chen K, Wen SY, Li J et al (2005) Pharmacokinetics of glimepiride and cytochrome P450 2 C9 genetic polymorphisms. Clin Pharmacol Ther 78(1):90–92
Niemi M, Cascorbi I, Timm R, Kroemer HK et al (2002) Glyburide and glimepiride pharmacokinetics in subjects with different CYP2C9 genotypes. Clin Pharmacol Ther 72(3):326–332
Kidd RS, Straughn AB, Meyer MC, Blaisdell J et al (1999) Pharmacokinetics of chlorpheniramine, phenytoin, glipizide and nifedipine in an individual homozygous for the CYP2C9*3 allele. Pharmacogenetics 9(1):71–80
Tomalik-Scharte D, Fuhr U, Hellmich M, Frank D et al (2011) Effect of the CYP2C8 genotype on the pharmacokinetics and pharmacodynamics of repaglinide. Drug Metab Dispos 39(5):927–932
Acknowledgements
The study has been financed by grants from the Swedish Research Council and the Scientific and Technological Research Council of Turkey.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gökalp, O., Gunes, A., Çam, H. et al. Mild hypoglycaemic attacks induced by sulphonylureas related to CYP2C9, CYP2C19 and CYP2C8 polymorphisms in routine clinical setting. Eur J Clin Pharmacol 67, 1223–1229 (2011). https://doi.org/10.1007/s00228-011-1078-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1078-4