Abstract
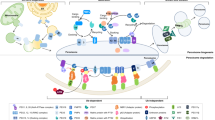
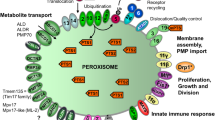
Peroxisomes and their (patho-)physiological importance in heath and disease have attracted increasing interest during last few decades. Together with mitochondria, peroxisomes comprise key metabolic platforms for oxidation of various fatty acids and redox regulation. In addition, peroxisomes contribute to bile acid, cholesterol, and plasmalogen biosynthesis. The importance of functional peroxisomes for cellular metabolism is demonstrated by the marked brain and systemic organ abnormalities occuring in peroxisome biogenesis disorders and peroxisomal enzyme deficiencies. Current evidences indicate that peroxisomal function is declined with aging, with peroxisomal dysfunction being linked to early onset of multiple age-related diseases including neurodegenerative diseases. Herein, we review recent progress toward understanding the physiological roles and pathological implications of peroxisomal dysfunctions, focusing on neurodegenerative disease.


Similar content being viewed by others
References
Abdel-Khalik J, Yutuc E, Crick PJ, Gustafsson JA, Warner M, Roman G, Talbot K, Gray E, Griffiths WJ, Turner MR, Wang Y (2017) Defective cholesterol metabolism in amyotrophic lateral sclerosis. J Lipid Res 58:267–278
Agrawal G, Subramani S (2016) De novo peroxisome biogenesis: evolving concepts and conundrums. Biochem Biophys Acta 1863:892–901
Amaral A, Castillo J, Estanyol JM, Ballesca JL, Ramalho-Santos J, Oliva R (2013) Human sperm tail proteome suggests new endogenous metabolic pathways. Mol Cell Proteom 12:330–342
Anding AL, Baehrecke EH (2017) Cleaning house: selective autophagy of organelles. Dev Cell 41:10–22
Antonenkov VD, Grunau S, Ohlmeier S, Hiltunen JK (2010) Peroxisomes are oxidative organelles. Antioxid Redox Signal 13:525–537
Astarita G, Jung KM, Berchtold NC, Nguyen VQ, Gillen DL, Head E, Cotman CW, Piomelli D (2010) Deficient liver biosynthesis of docosahexaenoic acid correlates with cognitive impairment in Alzheimer’s disease. PLoS ONE 5:e12538
Aubourg P, Wanders R (2013) Peroxisomal disorders. Handb Clin Neurol 113:1593–1609
Barberger-Gateau P, Letenneur L, Deschamps V, Peres K, Dartigues JF, Renaud S (2002) Fish, meat, and risk of dementia: cohort study. BMJ (Clin Res ed.) 325:932–933
Bar-On P, Crews L, Koob AO, Mizuno H, Adame A, Spencer B, Masliah E (2008) Statins reduce neuronal alpha-synuclein aggregation in in vitro models of Parkinson’s disease. J Neurochem 105:1656–1667
Bascoul-Colombo C, Guschina IA, Maskrey BH, Good M, O’Donnell VB, Harwood JL (2016) Dietary DHA supplementation causes selective changes in phospholipids from different brain regions in both wild type mice and the Tg2576 mouse model of Alzheimer’s disease. Biochem Biophys Acta 1861:524–537
Beel AJ, Sakakura M, Barrett PJ, Sanders CR (2010) Direct binding of cholesterol to the amyloid precursor protein: an important interaction in lipid-Alzheimer’s disease relationships? Biochem Biophys Acta 1801:975–982
Belkouch M, Hachem M, Elgot A, Van Lo A, Picq M, Guichardant M, Lagarde M, Bernoud-Hubac N (2016) The pleiotropic effects of omega-3 docosahexaenoic acid on the hallmarks of Alzheimer’s disease. J Nutr Biochem 38:1–11
Blandini F, Armentero MT (2012) Animal models of Parkinson’s disease. FEBS J 279:1156–1166
Bolner A, Micciolo R, Bosello O, Nordera GP (2016) A panel of oxidative stress markers in Parkinson’s disease. Clin Lab 62:105–112
Bonekamp NA, Volkl A, Fahimi HD, Schrader M (2009) Reactive oxygen species and peroxisomes: struggling for balance. BioFactors (Oxford, England) 35:346–355
Bourre JM (2004) Roles of unsaturated fatty acids (especially omega-3 fatty acids) in the brain at various ages and during ageing. J Nutr Health Aging 8:163–174
Boveris A, Oshino N, Chance B (1972) The cellular production of hydrogen peroxide. Biochem J 128:617–630
Bowers WE (1998) Christian de Duve and the discovery of lysosomes and peroxisomes. Trends Cell Biol 8:330–333
Braverman NE, Moser AB (2012) Functions of plasmalogen lipids in health and disease. Biochem Biophys Acta 1822:1442–1452
Braverman NE, D’Agostino MD, Maclean GE (2013) Peroxisome biogenesis disorders: biological, clinical and pathophysiological perspectives. Dev Disabil Res Rev 17:187–196
Breidert T, Callebert J, Heneka MT, Landreth G, Launay JM, Hirsch EC (2002) Protective action of the peroxisome proliferator-activated receptor-gamma agonist pioglitazone in a mouse model of Parkinson’s disease. J Neurochem 82:615–624
Brites P, Waterham HR, Wanders RJ (2004) Functions and biosynthesis of plasmalogens in health and disease. Biochem Biophys Acta 1636:219–231
Calder PC (2006) n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr 83:1505s–1519s
Calderon F, Kim HY (2004) Docosahexaenoic acid promotes neurite growth in hippocampal neurons. J Neurochem 90:979–988
Carrillo-Mora P, Luna R, Colin-Barenque L (2014) Amyloid beta: multiple mechanisms of toxicity and only some protective effects? Oxid Med Cell Longev 2014:795375
Cerri S, Blandini F (2018) Role of autophagy in Parkinson’s disease. Curr Med Chem. https://doi.org/10.2174/0929867325666180226094351
Chaturvedi RK, Beal MF (2008) PPAR: a therapeutic target in Parkinson’s disease. J Neurochem 106:506–518
Cheng D, Kim WS, Garner B (2008) Regulation of alpha-synuclein expression by liver X receptor ligands in vitro. NeuroReport 19:1685–1689
Cheng D, Jenner AM, Shui G, Cheong WF, Mitchell TW, Nealon JR, Kim WS, McCann H, Wenk MR, Halliday GM, Garner B (2011) Lipid pathway alterations in Parkinson’s disease primary visual cortex. PLoS ONE 6:e17299
Chen-Plotkin AS, Albin R, Alcalay R, Babcock D, Bajaj V, Bowman D, Buko A, Cedarbaum J, Chelsky D, Cookson, Dawson TM, Dewey R, Foroud T, Frasier M, German D, Gwinn K, Huang X, Kopil C, Kremer T, Lasch S, Marek K, Marto JA, Merchant K, Mollenhauer B, Naito A, Potashkin J, Reimer A, Rosenthal LS, Saunders-Pullman R, Scherzer CR, Sherer T, Singleton A, Sutherland M, Thiele I, van der Brug M, Van Keuren-Jensen K, Vaillancourt D, Walt D, West A, Zhang J (2018) Finding useful biomarkers for Parkinson’s disease. Sci Transl Med. https://doi.org/10.1126/scitranslmed.aam6003
Cherubini A, Andres-Lacueva C, Martin A, Lauretani F, Iorio AD, Bartali B, Corsi A, Bandinelli S, Mattson MP, Ferrucci L (2007) Low plasma N-3 fatty acids and dementia in older persons: the InCHIANTI study. J Gerontol Series A 62:1120–1126
Chinetti G, Fruchart JC, Staels B (2000) Peroxisome proliferator-activated receptors (PPARs): nuclear receptors at the crossroads between lipid metabolism and inflammation. Inflamm Res 49:497–505
Cho DH, Kim YS, Jo DS, Choe SK, Jo EK (2018) Pexophagy: molecular mechanisms and implications for health and diseases. Mol Cells 41:55–64
Chu BB, Liao YC, Qi W, Xie C, Du X, Wang J, Yang H, Miao HH, Li BL, Song BL (2015) Cholesterol transport through lysosome-peroxisome membrane contacts. Cell 161:291–306
Cipolla CM, Lodhi IJ (2017) Peroxisomal dysfunction in age-related diseases. Trends Endocrinol Metabol 28:297–308
Congdon EE, Sigurdsson EM (2018) Tau-targeting therapies for Alzheimer disease. Nat Rev Neurol 14:399–415
Conquer JA, Tierney MC, Zecevic J, Bettger WJ, Fisher RH (2000) Fatty acid analysis of blood plasma of patients with Alzheimer’s disease, other types of dementia, and cognitive impairment. Lipids 35:1305–1312
Cordeiro RM (2014) Reactive oxygen species at phospholipid bilayers: distribution, mobility and permeation. Biochem Biophys Acta 1838:438–444
Costello JL, Castro IG, Hacker C, Schrader TA, Metz J, Zeuschner D, Azadi AS, Godinho LF, Costina V, Findeisen P, Manner A, Islinger M, Schrader M (2017) ACBD5 and VAPB mediate membrane associations between peroxisomes and the ER. J Cell Biol 216:331–342
Crane DI (2014) Revisiting the neuropathogenesis of Zellweger syndrome. Neurochem Int 69:1–8
Deb R, Nagotu S (2017) Versatility of peroxisomes: an evolving concept. Tissue Cell 49:209–226
Deng BQ, Luo Y, Kang X, Li CB, Morisseau C, Yang J, Lee KSS, Huang J, Hu DY, Wu MY, Peng A, Hammock BD, Liu JY (2017) Epoxide metabolites of arachidonate and docosahexaenoate function conversely in acute kidney injury involved in GSK3beta signaling. Proc Natl Acad Sci USA 114:12608–12613
Deori NM, Kale A, Maurya PK, Nagotu S (2018) Peroxisomes: role in cellular ageing and age related disorders. Biogerontology 19:303–324
Di Cara F, Sheshachalam A, Braverman NE, Rachubinski RA, Simmonds AJ (2017) Peroxisome-mediated metabolism is required for immune response to microbial infection. Immunity 47:93–106.e107
Di Paolo G, Kim TW (2011) Linking lipids to Alzheimer’s disease: cholesterol and beyond. Nat Rev Neurosci 12:284–296
Dias V, Junn E, Mouradian MM (2013) The role of oxidative stress in Parkinson’s disease. J Parkinson’s Dis 3:461–491
Dixit E, Boulant S, Zhang Y, Lee AS, Odendall C, Shum B, Hacohen N, Chen ZJ, Whelan SP, Fransen M, Nibert ML, Superti-Furga G, Kagan JC (2010) Peroxisomes are signaling platforms for antiviral innate immunity. Cell 141:668–681
Doria M, Maugest L, Moreau T, Lizard G, Vejux A (2016) Contribution of cholesterol and oxysterols to the pathophysiology of Parkinson’s disease. Free Radic Biol Med 101:393–400
Dragonas C, Bertsch T, Sieber CC, Brosche T (2009) Plasmalogens as a marker of elevated systemic oxidative stress in Parkinson’s disease. Clin Chem Lab Med 47:894–897
Du J, Zhang L, Liu S, Zhang C, Huang X, Li J, Zhao N, Wang Z (2009) PPARgamma transcriptionally regulates the expression of insulin-degrading enzyme in primary neurons. Biochem Biophys Res Commun 383:485–490
Du Y, Wen Y, Guo X, Hao J, Wang W, He A, Fan Q, Li P, Liu L, Liang X, Zhang F (2018) A genome-wide expression association analysis identifies genes and pathways associated with amyotrophic lateral sclerosis. Cell Mol Neurobiol 38:635–639
Dubois V, Eeckhoute J, Lefebvre P, Staels B (2017) Distinct but complementary contributions of PPAR isotypes to energy homeostasis. J Clin Investig 127:1202–1214
Fabelo N, Martin V, Santpere G, Marin R, Torrent L, Ferrer I, Diaz M (2011) Severe alterations in lipid composition of frontal cortex lipid rafts from Parkinson’s disease and incidental Parkinson’s disease. Mol Medicine (Cambridge, Mass.) 17:1107–1118
Facciotti F, Ramanjaneyulu GS, Lepore M, Sansano S, Cavallari M, Kistowska M, Forss-Petter S, Ni G, Colone A, Singhal A, Berger J, Xia C, Mori L, De Libero G (2012) Peroxisome-derived lipids are self antigens that stimulate invariant natural killer T cells in the thymus. Nat Immunol 13:474–480
Fan J, Li X, Issop L, Culty M, Papadopoulos V (2016) ACBD2/ECI2-mediated peroxisome-mitochondria interactions in leydig cell steroid biosynthesis. Mol Endocrinol (Baltimore, Md.) 30:763–782
Fantini J, Carlus D, Yahi N (2011) The fusogenic tilted peptide (67-78) of alpha-synuclein is a cholesterol binding domain. Biochem Biophys Acta 1808:2343–2351
Farr RL, Lismont C, Terlecky SR, Fransen M (2016) Peroxisome biogenesis in mammalian cells: the impact of genes and environment. Biochem Biophys Acta 1863:1049–1060
Faust PL, Kovacs WJ (2014) Cholesterol biosynthesis and ER stress in peroxisome deficiency. Biochimie 98:75–85
Ferdinandusse S, Denis S, Faust PL, Wanders RJ (2009) Bile acids: the role of peroxisomes. J Lipid Res 50:2139–2147
Ferdinandusse S, Jimenez-Sanchez G, Koster J, Denis S, Van Roermund CW, Silva-Zolezzi I, Moser AB, Visser WF, Gulluoglu M, Durmaz O, Demirkol M, Waterham HR, Gokcay G, Wanders RJ, Valle D (2015) A novel bile acid biosynthesis defect due to a deficiency of peroxisomal ABCD3. Hum Mol Genet 24:361–370
Finkel T, Holbrook NJ (2000) Oxidants, oxidative stress and the biology of ageing. Nature 408:239–247
Fox MA, Nieuwesteeg MA, Willson JA, Cepeda M, Damjanovski S (2014) Knockdown of Pex11beta reveals its pivotal role in regulating peroxisomal genes, numbers, and ROS levels in Xenopus laevis A6 cells. In Vitro Cell Dev Biol Anim 50:340–349
Fransen M, Nordgren M, Wang B, Apanasets O (2012) Role of peroxisomes in ROS/RNS-metabolism: implications for human disease. Biochem Biophys Acta 1822:1363–1373
Fransen M, Lismont C, Walton P (2017) The peroxisome-mitochondria connection: how and why? Int J Mol Sci 18(6):1126
Frieden C, Garai K (2012) Structural differences between apoE3 and apoE4 may be useful in developing therapeutic agents for Alzheimer’s disease. Proc Natl Acad Sci USA 109:8913–8918
Giacobini E, Gold G (2013) Alzheimer disease therapy–moving from amyloid-beta to tau. Nat Rev Neurol 9:677–686
Ginsberg L, Rafique S, Xuereb JH, Rapoport SI, Gershfeld NL (1995) Disease and anatomic specificity of ethanolamine plasmalogen deficiency in Alzheimer’s disease brain. Brain Res 698:223–226
Graham WV, Bonito-Oliva A, Sakmar TP (2017) Update on Alzheimer’s disease therapy and prevention strategies. Annu Rev Med 68:413–430
Gray E, Rice C, Hares K, Redondo J, Kemp K, Williams M, Brown A, Scolding N, Wilkins A (2014) Reductions in neuronal peroxisomes in multiple sclerosis grey matter. Mult Scler (Houndmills, Basingstoke, England) 20:651–659
Green KN, Martinez-Coria H, Khashwji H, Hall EB, Yurko-Mauro KA, Ellis L, LaFerla FM (2007) Dietary docosahexaenoic acid and docosapentaenoic acid ameliorate amyloid-beta and tau pathology via a mechanism involving presenilin 1 levels. J Neurosci 27:4385–4395
Grimm MO, Grimm HS, Tomic I, Beyreuther K, Hartmann T, Bergmann C (2008) Independent inhibition of Alzheimer disease beta- and gamma-secretase cleavage by lowered cholesterol levels. J Biol Chem 283:11302–11311
Gudala K, Bansal D, Muthyala H (2013) Role of serum cholesterol in Parkinson’s disease: a meta-analysis of evidence. J Parkinson’s Dis 3:363–370
Guimaraes SC, Schuster M, Bielska E, Dagdas G, Kilaru S, Meadows BR, Schrader M, Steinberg G (2015) Peroxisomes, lipid droplets, and endoplasmic reticulum “hitchhike” on motile early endosomes. J Cell Biol 211:945–954
Guo X, Song W, Chen K, Chen X, Zheng Z, Cao B, Huang R, Zhao B, Wu Y, Shang HF (2015) The serum lipid profile of Parkinson’s disease patients: a study from China. Int J Neurosci 125:838–844
Hashimoto M, Shahdat HM, Katakura M, Tanabe Y, Gamoh S, Miwa K, Shimada T, Shido O (2009) Effects of docosahexaenoic acid on in vitro amyloid beta peptide 25-35 fibrillation. Biochem Biophys Acta 1791:289–296
Heneka MT, Carson MJ, El Khoury J, Landreth GE, Brosseron F, Feinstein DL, Jacobs AH, Wyss-Coray T, Vitorica J, Ransohoff RM, Herrup K, Frautschy SA, Finsen B, Brown GC, Verkhratsky A, Yamanaka K, Koistinaho J, Latz E, Halle A, Petzold GC, Town T, Morgan D, Shinohara ML, Perry VH, Holmes C, Bazan NG, Brooks DJ, Hunot S, Joseph B, Deigendesch N, Garaschuk O, Boddeke E, Dinarello CA, Breitner JC, Cole GM, Golenbock DT, Kummer MP (2015) Neuroinflammation in Alzheimer’s disease. Lancet Neurol 14:388–405
Hernandez DG, Reed X, Singleton AB (2016) Genetics in Parkinson disease: mendelian versus non-mendelian inheritance. J Neurochem 139(Suppl 1):59–74
Hiltunen JK, Mursula AM, Rottensteiner H, Wierenga RK, Kastaniotis AJ, Gurvitz A (2003) The biochemistry of peroxisomal beta-oxidation in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev 27:35–64
Hoefler G, Paschke E, Hoefler S, Moser AB, Moser HW (1991) Photosensitized killing of cultured fibroblasts from patients with peroxisomal disorders due to pyrene fatty acid-mediated ultraviolet damage. J Clin Investig 88:1873–1879
Hohn A, Grune T (2013) Lipofuscin: formation, effects and role of macroautophagy. Redox Biol 1:140–144
Hooijmans CR, Van der Zee CE, Dederen PJ, Brouwer KM, Reijmer YD, van Groen T, Broersen LM, Lutjohann D, Heerschap A, Kiliaan AJ (2009) DHA and cholesterol containing diets influence Alzheimer-like pathology, cognition and cerebral vasculature in APPswe/PS1dE9 mice. Neurobiol Dis 33:482–498
Hossain S, Hashimoto M, Katakura M, Miwa K, Shimada T, Shido O (2009) Mechanism of docosahexaenoic acid-induced inhibition of in vitro Abeta1-42 fibrillation and Abeta1-42-induced toxicity in SH-S5Y5 cells. J Neurochem 111:568–579
Hu J, Baker A, Bartel B, Linka N, Mullen RT, Reumann S, Zolman BK (2012) Plant peroxisomes: biogenesis and function. Plant Cell 24:2279–2303
Hua R, Cheng D, Coyaud E, Freeman S, Di Pietro E, Wang Y, Vissa A, Yip CM, Fairn GD, Braverman N, Brumell JH, Trimble WS, Raught B, Kim PK (2017) VAPs and ACBD5 tether peroxisomes to the ER for peroxisome maintenance and lipid homeostasis. J Cell Biol 216:367–377
Hwang O (2013) Role of oxidative stress in Parkinson’s disease. Exp Neurobiol 22:11–17
Hwang I, Lee J, Huh JY, Park J, Lee HB, Ho YS, Ha H (2012) Catalase deficiency accelerates diabetic renal injury through peroxisomal dysfunction. Diabetes 61:728–738
Islinger M, Cardoso MJ, Schrader M (2010) Be different–the diversity of peroxisomes in the animal kingdom. Biochem Biophys Acta 1803:881–897
Islinger M, Voelkl A, Fahimi HD, Schrader M (2018) The peroxisome: an update on mysteries 2.0. Histochem Cell Biol 150:443–471
Issemann I, Green S (1990) Activation of a member of the steroid hormone receptor superfamily by peroxisome proliferators. Nature 347:645–650
Jenner P (2003) Oxidative stress in Parkinson’s disease. Ann Neurol 53(Suppl 3):S26–S36 discussion S36-28
Jenner P, Olanow CW (1996) Oxidative stress and the pathogenesis of Parkinson’s disease. Neurology 47:S161–170
Jiang T, Sun Q, Chen S (2016) Oxidative stress: a major pathogenesis and potential therapeutic target of antioxidative agents in Parkinson’s disease and Alzheimer’s disease. Prog Neurobiol 147:1–19
Kalmijn S, Launer LJ, Ott A, Witteman JC, Hofman A, Breteler MM (1997) Dietary fat intake and the risk of incident dementia in the Rotterdam Study. Ann Neurol 42:776–782
Kamat PK, Kalani A, Rai S, Swarnkar S, Tota S, Nath C, Tyagi N (2016) Mechanism of oxidative stress and synapse dysfunction in the pathogenesis of Alzheimer’s disease: understanding the therapeutics strategies. Mol Neurobiol 53:648–661
Kim JY, Jang A, Reddy R, Yoon WH, Jankowsky JL (2016) Neuronal overexpression of human VAPB slows motor impairment and neuromuscular denervation in a mouse model of ALS. Hum Mol Genet 25:4661–4673
Koch J, Pranjic K, Huber A, Ellinger A, Hartig A, Kragler F, Brocard C (2010) PEX11 family members are membrane elongation factors that coordinate peroxisome proliferation and maintenance. J Cell Sci 123:3389–3400
Koob AO, Ubhi K, Paulsson JF, Kelly J, Rockenstein E, Mante M, Adame A, Masliah E (2010) Lovastatin ameliorates alpha-synuclein accumulation and oxidation in transgenic mouse models of alpha-synucleinopathies. Exp Neurol 221:267–274
Kou J, Kovacs GG, Hoftberger R, Kulik W, Brodde A, Forss-Petter S, Honigschnabl S, Gleiss A, Brugger B, Wanders R, Just W, Budka H, Jungwirth S, Fischer P, Berger J (2011) Peroxisomal alterations in Alzheimer’s disease. Acta Neuropathol 122:271–283
Kovacs WJ, Tape KN, Shackelford JE, Duan X, Kasumov T, Kelleher JK, Brunengraber H, Krisans SK (2007) Localization of the pre-squalene segment of the isoprenoid biosynthetic pathway in mammalian peroxisomes. Histochem Cell Biol 127:273–290
LaFerla FM, Green KN, Oddo S (2007) Intracellular amyloid-beta in Alzheimer’s disease. Nat Rev Neurosci 8:499–509
Landreth G (2007) Therapeutic use of agonists of the nuclear receptor PPARgamma in Alzheimer’s disease. Curr Alzheimer Res 4:159–164
Lang A, John Peter AT, Kornmann B (2015) ER-mitochondria contact sites in yeast: beyond the myths of ERMES. Curr Opin Cell Biol 35:7–12
Legakis JE, Koepke JI, Jedeszko C, Barlaskar F, Terlecky LJ, Edwards HJ, Walton PA, Terlecky SR (2002) Peroxisome senescence in human fibroblasts. Mol Biol Cell 13:4243–4255
Lim SY, Suzuki H (2000) Intakes of dietary docosahexaenoic acid ethyl ester and egg phosphatidylcholine improve maze-learning ability in young and old mice. J Nutr 130:1629–1632
Lizard G, Rouaud O, Demarquoy J, Cherkaoui-Malki M, Iuliano L (2012) Potential roles of peroxisomes in Alzheimer’s disease and in dementia of the Alzheimer’s type. J Alzheimer’s Dis 29:241–254
Lodhi IJ, Wei X, Yin L, Feng C, Adak S, Abou-Ezzi G, Hsu FF, Link DC, Semenkovich CF (2015) Peroxisomal lipid synthesis regulates inflammation by sustaining neutrophil membrane phospholipid composition and viability. Cell Metab 21:51–64
Lukiw WJ, Cui JG, Marcheselli VL, Bodker M, Botkjaer A, Gotlinger K, Serhan CN, Bazan NG (2005) A role for docosahexaenoic acid-derived neuroprotectin D1 in neural cell survival and Alzheimer disease. J Clin Investig 115:2774–2783
Ma QL, Yang F, Rosario ER, Ubeda OJ, Beech W, Gant DJ, Chen PP, Hudspeth B, Chen C, Zhao Y, Vinters HV, Frautschy SA, Cole GM (2009) Beta-amyloid oligomers induce phosphorylation of tau and inactivation of insulin receptor substrate via c-Jun N-terminal kinase signaling: suppression by omega-3 fatty acids and curcumin. J Neurosci 29:9078–9089
Marchetti DP, Donida B, Jacques CE, Deon M, Hauschild TC, Koehler-Santos P, de Moura Coelho D, Coitinho AS, Jardim LB, Vargas CR (2018) Inflammatory profile in X-linked adrenoleukodystrophy patients: understanding disease progression. J Cell Biochem 119:1223–1233
Martin MG, Pfrieger F, Dotti CG (2014) Cholesterol in brain disease: sometimes determinant and frequently implicated. EMBO Rep 15:1036–1052
Marwarha G, Rhen T, Schommer T, Ghribi O (2011) The oxysterol 27-hydroxycholesterol regulates alpha-synuclein and tyrosine hydroxylase expression levels in human neuroblastoma cells through modulation of liver X receptors and estrogen receptors–relevance to Parkinson’s disease. J Neurochem 119:1119–1136
Mattiazzi Usaj M, Brloznik M, Kaferle P, Zitnik M, Wolinski H, Leitner F, Kohlwein SD, Zupan B, Petrovic U (2015) Genome-wide localization study of yeast Pex11 identifies peroxisome-mitochondria interactions through the ERMES complex. J Mol Biol 427:2072–2087
McGuinness MC, Griffin DE, Raymond GV, Washington CA, Moser HW, Smith KD (1995) Tumor necrosis factor-alpha and X-linked adrenoleukodystrophy. J Neuroimmunol 61:161–169
Mitchell J, Paul P, Chen HJ, Morris A, Payling M, Falchi M, Habgood J, Panoutsou S, Winkler S, Tisato V, Hajitou A, Smith B, Vance C, Shaw C, Mazarakis ND, de Belleroche J (2010) Familial amyotrophic lateral sclerosis is associated with a mutation in D-amino acid oxidase. Proc Natl Acad Sci USA 107:7556–7561
Miville-Godbout E, Bourque M, Morissette M, Al-Sweidi S, Smith T, Mochizuki A, Senanayake V, Jayasinghe D, Wang L, Goodenowe D, Di Paolo T (2016) Plasmalogen augmentation reverses striatal dopamine loss in MPTP mice. PLoS ONE 11:e0151020
Miville-Godbout E, Bourque M, Morissette M, Al-Sweidi S, Smith T, Jayasinghe D, Ritchie S, Di Paolo T (2017) Plasmalogen precursor mitigates striatal dopamine loss in MPTP mice. Brain Res 1674:70–76
Miyazaki C, Saitoh M, Itoh M, Yamashita S, Miyagishi M, Takashima S, Moser AB, Iwamori M, Mizuguchi M (2013) Altered phospholipid molecular species and glycolipid composition in brain, liver and fibroblasts of Zellweger syndrome. Neurosci Lett 552:71–75
Morita M, Kurochkin IV, Motojima K, Goto S, Takano T, Okamura S, Sato R, Yokota S, Imanaka T (2000) Insulin-degrading enzyme exists inside of rat liver peroxisomes and degrades oxidized proteins. Cell Struct Funct 25:309–315
Murphy S, Martin S, Parton RG (2009) Lipid droplet-organelle interactions; sharing the fats. Biochem Biophys Acta 1791:441–447
Oku M, Sakai Y (2010) Peroxisomes as dynamic organelles: autophagic degradation. FEBS J 277:3289–3294
Pan Y, Khalil H, Nicolazzo JA (2015) The impact of docosahexaenoic acid on Alzheimer’s disease: is there a role of the blood-brain barrier? Curr Clin Pharmacol 10:222–241
Paul P, de Belleroche J (2012) The role of D-amino acids in amyotrophic lateral sclerosis pathogenesis: a review. Amino Acids 43:1823–1831
Pomatto LC, Raynes R, Davies KJ (2017) The peroxisomal Lon protease LonP2 in aging and disease: functions and comparisons with mitochondrial Lon protease LonP1. Biol Rev Camb Philos Soc 92:739–753
Popp J, Meichsner S, Kolsch H, Lewczuk P, Maier W, Kornhuber J, Jessen F, Lutjohann D (2013) Cerebral and extracerebral cholesterol metabolism and CSF markers of Alzheimer’s disease. Biochem Pharmacol 86:37–42
Prasanthi JR, Huls A, Thomasson S, Thompson A, Schommer E, Ghribi O (2009) Differential effects of 24-hydroxycholesterol and 27-hydroxycholesterol on beta-amyloid precursor protein levels and processing in human neuroblastoma SH-SY5Y cells. Mol Neurodegener 4:1
Prinz WA (2014) Bridging the gap: membrane contact sites in signaling, metabolism, and organelle dynamics. J Cell Biol 205:759–769
Puspita L, Chung SY, Shim JW (2017) Oxidative stress and cellular pathologies in Parkinson’s disease. Mol Brain 10:53
Quinn LP, Crook B, Hows ME, Vidgeon-Hart M, Chapman H, Upton N, Medhurst AD, Virley DJ (2008) The PPARgamma agonist pioglitazone is effective in the MPTP mouse model of Parkinson’s disease through inhibition of monoamine oxidase B. Br J Pharmacol 154:226–233
Ramsay RR, Zammit VA (2004) Carnitine acyltransferases and their influence on CoA pools in health and disease. Mol Aspects Med 25:475–493
Robson LG, Dyall S, Sidloff D, Michael-Titus AT (2010) Omega-3 polyunsaturated fatty acids increase the neurite outgrowth of rat sensory neurones throughout development and in aged animals. Neurobiol Aging 31:678–687
Sacchi S, Cappelletti P, Murtas G (2018) Biochemical properties of human D-amino acid oxidase variants and their potential significance in pathologies. Front Mol Biosci 5:55
Sandalio LM, Romero-Puertas MC (2015) Peroxisomes sense and respond to environmental cues by regulating ROS and RNS signalling networks. Ann Bot 116:475–485
Schrader M, Godinho LF, Costello JL, Islinger M (2015) The different facets of organelle interplay-an overview of organelle interactions. Front Cell Dev Biol 3:56
Shadfar S, Hwang CJ, Lim MS, Choi DY, Hong JT (2015) Involvement of inflammation in Alzheimer’s disease pathogenesis and therapeutic potential of anti-inflammatory agents. Arch Pharmacal Res 38:2106–2119
Shai N, Yifrach E, van Roermund CWT, Cohen N, Bibi C, IJ L, Cavellini L, Meurisse J, Schuster R, Zada L, Mari MC, Reggiori FM, Hughes AL, Escobar-Henriques M, Cohen MM, Waterham HR, Wanders RJA, Schuldiner M, Zalckvar E (2018) Systematic mapping of contact sites reveals tethers and a function for the peroxisome-mitochondria contact. Nat Commun 9:1761
Sheykhansari S, Kozielski K, Bill J, Sitti M, Gemmati D, Zamboni P, Singh AV (2018) Redox metals homeostasis in multiple sclerosis and amyotrophic lateral sclerosis: a review. Cell Death Dis 9:348
Soderberg M, Edlund C, Kristensson K, Dallner G (1991) Fatty acid composition of brain phospholipids in aging and in Alzheimer’s disease. Lipids 26:421–425
Sugiura A, Mattie S, Prudent J, McBride HM (2017) Newly born peroxisomes are a hybrid of mitochondrial and ER-derived pre-peroxisomes. Nature 542:251–254
Tan LC, Methawasin K, Tan EK, Tan JH, Au WL, Yuan JM, Koh WP (2016) Dietary cholesterol, fats and risk of Parkinson’s disease in the Singapore Chinese Health Study. J Neurol Neurosurg Psychiatry 87:86–92
Thenganatt MA, Jankovic J (2014) Parkinson disease subtypes. JAMA Neurol 71:499–504
Trompier D, Vejux A, Zarrouk A, Gondcaille C, Geillon F, Nury T, Savary S, Lizard G (2014) Brain peroxisomes. Biochimie 98:102–110
Tully AM, Roche HM, Doyle R, Fallon C, Bruce I, Lawlor B, Coakley D, Gibney MJ (2003) Low serum cholesteryl ester-docosahexaenoic acid levels in Alzheimer’s disease: a case-control study. Br J Nutr 89:483–489
Walbrecq G, Wang B, Becker S, Hannotiau A, Fransen M, Knoops B (2015) Antioxidant cytoprotection by peroxisomal peroxiredoxin-5. Free Radic Biol Med 84:215–226
Wanders RJ (2014) Metabolic functions of peroxisomes in health and disease. Biochimie 98:36–44
Wanders RJ, Waterham HR, Ferdinandusse S (2015) Metabolic interplay between peroxisomes and other subcellular organelles including mitochondria and the endoplasmic reticulum. Front Cell Dev Biol 3:83
Wang W, Shinto L, Connor WE, Quinn JF (2008) Nutritional biomarkers in Alzheimer’s disease: the association between carotenoids, n-3 fatty acids, and dementia severity. J Alzheimer’s Dis 13:31–38
Wang X, Wang Z, Liu JZ, Hu JX, Chen HL, Li WL, Hai CX (2011) Double antioxidant activities of rosiglitazone against high glucose-induced oxidative stress in hepatocyte. Toxicol In Vitro 25:839–847
Wang S, Horn PJ, Liou LC, Muggeridge MI, Zhang Z, Chapman KD, Witt SN (2013) A peroxisome biogenesis deficiency prevents the binding of alpha-synuclein to lipid droplets in lipid-loaded yeast. Biochem Biophys Res Commun 438:452–456
Williams C, Opalinski L, Landgraf C, Costello J, Schrader M, Krikken AM, Knoops K, Kram AM, Volkmer R, van der Klei IJ (2015) The membrane remodeling protein Pex11p activates the GTPase Dnm1p during peroxisomal fission. Proc Natl Acad Sci USA 112:6377–6382
Wolozin B, Wang SW, Li NC, Lee A, Lee TA, Kazis LE (2007) Simvastatin is associated with a reduced incidence of dementia and Parkinson’s disease. BMC Med 5:20
Xiao Z, Wang J, Chen W, Wang P, Zeng H, Chen W (2012) Association studies of several cholesterol-related genes (ABCA1, CETP and LIPC) with serum lipids and risk of Alzheimer’s disease. Lipids Health Dis 11:163
Xu Z, Asahchop EL, Branton WG, Gelman BB, Power C, Hobman TC (2017) MicroRNAs upregulated during HIV infection target peroxisome biogenesis factors: implications for virus biology, disease mechanisms and neuropathology. PLoS Pathog 13:e1006360
Xue-Shan Z, Juan P, Qi W, Zhong R, Li-Hong P, Zhi-Han T, Zhi-Sheng J, Gui-Xue W, Lu-Shan L (2016) Imbalanced cholesterol metabolism in Alzheimer’s disease. Clin Chim Acta 456:107–114
Ye S, Huang Y, Mullendorff K, Dong L, Giedt G, Meng EC, Cohen FE, Kuntz ID, Weisgraber KH, Mahley RW (2005) Apolipoprotein (apo) E4 enhances amyloid beta peptide production in cultured neuronal cells: apoE structure as a potential therapeutic target. Proc Natl Acad Sci USA 102:18700–18705
Zhang SO, Trimble R, Guo F, Mak HY (2010) Lipid droplets as ubiquitous fat storage organelles in C. elegans. BMC Cell Biol 11:96
Zhang J, Tripathi DN, Jing J, Alexander A, Kim J, Powell RT, Dere R, Tait-Mulder J, Lee JH, Paull TT, Pandita RK, Charaka VK, Pandita TK, Kastan MB, Walker CL (2015) ATM functions at the peroxisome to induce pexophagy in response to ROS. Nat Cell Biol 17:1259–1269
Zhang Y, Yan T, Sun D, Xie C, Zheng Y, Zhang L, Yagai T, Krausz KW, Bisson WH, Yang X, Gonzalez FJ (2018) Structure-activity relationships of the main bioactive constituents of euodia rutaecarpa on aryl hydrocarbon receptor activation and associated bile acid homeostasis. Drug Metabol Dispos 46:1030–1040
Zhao Y, Calon F, Julien C, Winkler JW, Petasis NA, Lukiw WJ, Bazan NG (2011) Docosahexaenoic acid-derived neuroprotectin D1 induces neuronal survival via secretase- and PPARgamma-mediated mechanisms in Alzheimer’s disease models. PLoS ONE 6:e15816
Zhou P, Chen Z, Zhao N, Liu D, Guo ZY, Tan L, Hu J, Wang Q, Wang JZ, Zhu LQ (2011) Acetyl-L-carnitine attenuates homocysteine-induced Alzheimer-like histopathological and behavioral abnormalities. Rejuvenation Res 14:669–679
Zhu L, Zhong M, Elder GA, Sano M, Holtzman DM, Gandy S, Cardozo C, Haroutunian V, Robakis NK, Cai D (2015) Phospholipid dysregulation contributes to ApoE4-associated cognitive deficits in Alzheimer’s disease pathogenesis. Proc Natl Acad Sci USA 112:11965–11970
Zoeller RA, Lake AC, Nagan N, Gaposchkin DP, Legner MA, Lieberthal W (1999) Plasmalogens as endogenous antioxidants: somatic cell mutants reveal the importance of the vinyl ether. Biochem J 338:769–776
Acknowledgements
This research was supported by a Grant of the Korea–UK Collaborative Alzheimer’s Disease Research Project by Ministry of Health & Welfare, Republic of Korea (HI14C1913), and supported by National Research Foundation of Korea funded by the Ministry of Science & ICT (2017R1A2B4005501).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jo, D.S., Cho, DH. Peroxisomal dysfunction in neurodegenerative diseases. Arch. Pharm. Res. 42, 393–406 (2019). https://doi.org/10.1007/s12272-019-01131-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-019-01131-2