Abstract
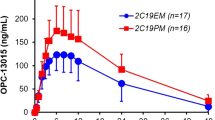
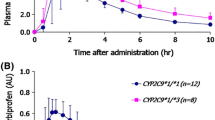
Celecoxib, a selective cyclooxygenase (COX)-2 inhibitor, is used for the treatment of rheumatoid arthritis and osteoarthritis. The predominant hepatic metabolism of celecoxib to celecoxib carboxylic acid (CCA) is mediated mainly by CYP2C9. We investigated the effects of the major CYP2C9 genetic variants in Asian populations, CYP2C9*3 and CYP2C9*13, on the pharmacokinetics of celecoxib and its carboxylic acid metabolite in healthy Korean subjects. A single 200-mg oral dose of celecoxib was given to 52 Korean subjects with different CYP2C9 genotypes: CYP2C9EM (n = 26; CYP2C9*1/*1), CYP2C9IM (n = 24; CYP2C9*1/*3 and *1/*13), and CYP2C9PM (n = 2; CYP2C9*3/*3). Celecoxib and CCA concentrations in plasma samples collected up to 48 or 96 h after drug intake were determined by HPLC–MS/MS. The mean area under the plasma concentration–time curve (AUC0–∞) of celecoxib was increased 1.63-fold (P < 0.001), and the apparent oral clearance (CL/F) of celecoxib was decreased by 39.6% in the CYP2C9IM genotype group compared with that of CYP2C9EM (P < 0.001). The overall pharmacokinetic parameters for celecoxib in CYP2C9*1/*13 subjects were similar to those in CYP2C9*1/*3 subjects. Two subjects with CYP2C9PM genotype both showed markedly higher AUC0–∞, prolonged half-life, and lower CL/F for celecoxib than did subjects with CYP2C9EM and IM genotypes. CYP2C9*3 and CYP2C9*13 variant alleles significantly affected the plasma concentration of celecoxib.





Similar content being viewed by others
References
Bae JW, Kim HK, Kim JH, Yang SI, Kim MJ, Jang CG, Park YS, Lee SY (2005) Allele and genotype frequencies of CYP2C9 in a Korean population. Br J Clin Pharmacol 60:418–422
Bae JW, Choi CI, Jang CG, Lee SY (2011a) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71:550–555
Bae JW, Choi CI, Kim MJ, Oh DH, Keum SK, Park JI, Kim BH, Bang HK, Oh SG, Kang BS, Park HJ, Kim HD, Ha JH, Shin HJ, Kim YH, Na HS, Chung MW, Jang CG, Lee SY (2011b) Frequency of CYP2C9 alleles in Koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin 32:1303–1308
Bae JW, Choi CI, Lee HI, Lee YJ, Jang CG, Lee SY (2012) Effects of CYP2C9*1/*3 and *1/*13 on the pharmacokinetics of losartan and its active metabolite E-3174. Int J Clin Pharmacol Ther 50:683–689
Brenner SS, Herrlinger C, Dilger K, Mürdter TE, Hofmann U, Marx C, Klotz U (2003) Influence of age and cytochrome P450 2C9 genotype on the steady-state disposition of diclofenac and celecoxib. Clin Pharmacokinet 42:283–292
Byeon JY, Kim YH, Na HS, Jang JH, Kim SH, Lee YJ, Bae JW, Kim IS, Jang CG, Chung MW, Lee SY (2015) Effects of the CYP2D6*10 allele on the pharmacokinetics of atomoxetine and its metabolites. Arch Pharm Res 38:2083–2091
Caldwell B, Aldington S, Weatherall M, Shirtcliffe P, Beasley R (2006) Risk of cardiovascular events and celecoxib: a systematic review and meta-analysis. J R Soc Med 99:132–140
Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109:476–480
Choi CI, Kim MJ, Chung EK, Lee HI, Jang CG, Bae JW, Lee SY (2012) CYP2C9*3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol 68:149–154
Davies NM, McLachlan AJ, Day RO, Williams KM (2000) Clinical pharmacokinetics and pharmacodynamics of celecoxib: a selective cyclo-oxygenase-2 inhibitor. Clin Pharmacokinet 38:225–242
Dupont WD, Plummer WD Jr (1998) Power and sample size calculations for studies involving linear regression. Control Clin Trials 19:589–601
García-Martín E, Martínez C, Ladero JM, Agúndez JA (2006) Interethnic and intraethnic variability of CYP2C8 and CYP2C9 polymorphisms in healthy individuals. Mol Diagn Ther 10:29–40
Guo Y, Zhang Y, Wang Y, Chen X, Si D, Zhong D, Fawcett JP, Zhou H (2005a) Role of CYP2C9 and its variants (CYP2C9*3 and CYP2C9*13) in the metabolism of lornoxicam in humans. Drug Metab Dispos 33:749–753
Guo Y, Wang Y, Si D, Fawcett PJ, Zhong D, Zhou H (2005b) Catalytic activities of human cytochrome P450 2C9*1, 2C9*3 and 2C9*13. Xenobiotica 35:853–861
Kirchheiner J, Brockmöller J (2005) Clinical consequences of cytochrome P450 2C9 polymorphisms. Clin Pharmacol Ther 77:1–16
Kirchheiner J, Störmer E, Meisel C, Steinbach N, Roots I, Brockmöller J (2003a) Influence of CYP2C9 genetic polymorphisms on pharmacokinetics of celecoxib and its metabolites. Pharmacogenetics 13:473–480
Kirchheiner J, Meineke I, Steinbach N, Meisel C, Roots I, Brockmöller J (2003b) Pharmacokinetics of diclofenac and inhibition of cyclooxygenases 1 and 2: no relationship to the CYP2C9 genetic polymorphism in humans. Br J Clin Pharmacol 55:51–61
Lee CR, Goldstein JA, Pieper JA (2002) Cytochrome P450 2C9 polymorphisms: a comprehensive review of the in vitro and human data. Pharmacogenetics 12:251–263
Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA (2003a) Tolbutamide, flurbiprofen, and losartan as probes of CYP2C9 activity in humans. J Clin Pharmacol 43:84–91
Lee CR, Pieper JA, Frye RF, Hinderliter AL, Blaisdell JA, Goldstein JA (2003b) Differences in flurbiprofen pharmacokinetics between CYP2C9*1/*1, *1/*2, and *1/*3 genotypes. Eur J Clin Pharmacol 58:791–794
Lee HI, Bae JW, Choi CI, Lee YJ, Byeon JY, Jang CG, Lee SY (2014) Strongly increased exposure of meloxicam in CYP2C9*3/*3 individuals. Pharmacogenet Genom 24:113–117
Lee YJ, Byeon JY, Kim YH, Kim SH, Choi CI, Bae JW, Sohn UD, Jang CG, Lee J, Lee SY (2015) Effects of CYP2C9*1/*3 genotype on the pharmacokinetics of flurbiprofen in Korean subjects. Arch Pharm Res 38:1232–1237
Lee HJ, Kim YH, Kim SH, Lee CM, Yang AY, Jang CG, Lee SY, Bae JW, Choi CI (2016) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of zafirlukast. Arch Pharm Res 39:1013–1019
Li Z, Wang G, Wang LS, Zhang W, Tan ZR, Fan L, Chen BL, Li Q, Liu J, Tu JH, Hu DL, Liu ZQ, Zhou HH (2009) Effects of the CYP2C9*13 allele on the pharmacokinetics of losartan in healthy male subjects. Xenobiotica 39:788–793
Liu YL, Zhang W, Tan ZR, Ouyang DS, Luo CH, Liu ZQ, Qiu Y, Chen Y, He YJ, Zhou G, Zhou HH (2006) Effect of the CYP2C9*3 allele on lornoxicam metabolism. Clin Chim Acta 364:287–291
Liu R, Gong C, Tao L, Yang W, Zheng X, Ma P, Ding L (2015) Influence of genetic polymorphisms on the pharmacokinetics of celecoxib and its two main metabolites in healthy Chinese subjects. Eur J Pharm Sci 79:13–19
Lundbald MS, Ohlsson S, Johansson P, Lafolie P, Eliasson E (2006) Accumulation of celecoxib with a 7-fold higher drug exposure in individuals homozygous for CYP2C9*3. Clin Pharmacol Ther 79:287–288
Maekawa K, Harakawa N, Sugiyama E, Tohkin M, Kim SR, Kaniwa N, Katori N, Hasegawa R, Yasuda K, Kamide K, Miyata T, Saito Y, Sawada J (2009) Substrate-dependent functional alterations of seven CYP2C9 variants found in Japanese subjects. Drug Metab Dispos 37:1895–1903
Martin JH, Begg EJ, Kennedy MA, Roberts R, Barclay ML (2001) Is cytochrome P450 2C9 genotype associated with NSAID gastric ulceration? Br J Clin Pharmacol 51:627–630
Martinez C, Blanco G, Ladero JM, Garcia-Martin E, Taxonera C, Gamito FG, Diaz-Rubio M, Agundez JA (2004) Genetic predisposition to acute gastrointestinal bleeding after NSAIDs use. Br J Pharmacol 141:205–208
Miners JO, Birkett DJ (1998) Cytochrome P4502C9: an enzyme of major importance in human drug metabolism. Br J Clin Pharmacol 51:1003–1008
Mohammed S, Croom DW (1999) Gastropathy due to celecoxib, a cyclooxygenase-2 inhibitor. N Engl J Med 340:2005–2006
Moore RA, Derry S, Makinson GT, McQuay HJ (2005) Tolerability and adverse events in clinical trials of celecoxib in osteoarthritis and rheumatoid arthritis: systematic review and meta-analysis of information from company clinical trial reports. Arthritis Res Ther 7:R644–665
Nakai K, Habano W, Nakai K, Fukushima N, Suwabe A, Moriya S, Osano K, Gurwitz D (2005) Ethnic differences in CYP2C9*2 (Arg144Cys) and CYP2C9*3 (Ile359Leu) genotypes in Japanese and Israeli populations. Life Sci 78:107–111
Paulson SK, Hribar JD, Liu NW, Hajdu E, Bible RH Jr, Piergies A, Karim A (2000) Metabolism and excretion of [14C]celecoxib in healthy male volunteers. Drug Metab Dispos 28:308–314
Perini JA, Vianna-Jorge R, Brogliato AR, Suarez-Kurtz G (2005) Influence of CYP2C9 genotypes on the pharmacokinetics and pharmacodynamics of piroxicam. Clin Pharmacol Ther 78:362–369
Prieto-Pérez R, Ochoa D, Cabaleiro T, Román M, Sánchez-Rojas SD, Talegón M, Abad-Santos F (2013) Evaluation of the relationship between polymorphisms in CYP2C8 and CYP2C9 and the pharmacokinetics of celecoxib. J Clin Pharmacol 53:1261–1267
Rodrigues AD (1999) Integrated cytochrome P450 reaction phenotyping: attempting to bridge the gap between cDNA-expressed cytochromes P450 and native human liver microsomes. Biochem Pharmacol 57:465–480
Sandberg M, Yasar U, Strömberg P, Höög JO, Eliasson E (2002) Oxidation of celecoxib by polymorphic cytochrome P450 2C9 and alcohol dehydrogenase. Br J Clin Pharmacol 54:423–429
Scott SA, Khasawneh R, Peter I, Kornreich R, Desnick RJ (2011) Combined CYP2C9, VKORC1 and CYP4F2 frequencies among racial and ethnic groups. Pharmacogenomics 11:781–791
Si D, Guo Y, Zhang Y, Yang L, Zhou H, Zhong D (2004) Identification of a novel variant CYP2C9 allele in Chinese. Pharmacogenetics 14:465–469
Stempak D, Bukaveckas BL, Linder M, Koren G, Baruchel S (2005) Cytochrome P450 2C9 genotype: impact on celecoxib safety and pharmacokinetics in a pediatric patient. Clin Pharmacol Ther 78:309–310
Tang C, Shou M, Mei Q, Rushmore TH, Rodrigues AD (2000) Major role of human liver microsomal cytochrome P450 2C9 (CYP2C9) in the oxidative metabolism of celecoxib, a novel cyclooxygenase-II inhibitor. J Pharmacol Exp Ther 293:453–459
Vienna-Jorge R, Perini JA, Rondinelli E, Suarez-Kurtz G (2004) CYP2C9 genotypes and the pharmacokinetics of tenoxicam in Brazilians. Clin Pharmacol Ther 76:18–26
Wynne HA, Long A, Nicholson E, Ward A, Keir D (1998) Are altered pharmacokinetics of nonsteroidal anti-inflammatory drugs (NSAIDs) a risk factor for gastrointestinal bleeding? Br J Clin Pharmacol 45:405–408
Zhang Y, Zhong D, Si D, Guo Y, Chen X, Zhou H (2005) Lornoxicam pharmacokinetics in relation to cytochrome P450 2C9 genotype. Br J Clin Pharmacol 59:14–17
Zhou YH, Zheng QC, Li ZS, Zhang Y, Sun M, Sun CC, Si D, Cai L, Guo Y, Zhou H (2006) On the human CYP2C9*13 variant activity reduction: a molecular dynamics simulation and docking study. Biochimie 88:1457–1465
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2016R1A2B4007381).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no potential conflict of interest with respect to the authorship and/or publication of this article.
Rights and permissions
About this article
Cite this article
Kim, SH., Kim, DH., Byeon, JY. et al. Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch. Pharm. Res. 40, 382–390 (2017). https://doi.org/10.1007/s12272-016-0861-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-016-0861-2