Abstract
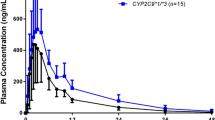
Meloxicam, a non-steroidal anti-inflammatory drug, is used for the treatment of rheumatoid arthritis and osteoarthritis. Cytochrome P450 (CYP) 2C9 and CYP3A4 are major and minor enzymes involved in the metabolism of meloxicam. Impaired enzyme activity of CYP2C9 variants increases the plasma exposures of meloxicam and the risk of adverse events. The objective of our study is to develop and validate the physiologically based pharmacokinetic (PBPK) model of meloxicam related to CYP2C9 genetic polymorphism using the PK-Sim® software. In vitro kcat of CYP2C9 was optimized in different CYP2C9 genotypes. The demographic and pharmacokinetic dataset for the development of the PBPK model was extracted from two previous clinical pharmacokinetic studies. Thirty-one clinical datasets, representing different dose regimens and demographic characteristics, were utilized to validate the PBPK model. The shapes of simulated plasma concentration–time profiles in each CYP2C9 genotype were visually similar to observed profiles. The predicted exposures (AUCinf) of meloxicam in CYP2C9*1/*3, CYP2C9*1/*13, and CYP2C9*3/*3 genotypes were increased by 1.77-, 2.91-, and 8.35-fold compared to CYP2C9*1/*1 genotype, respectively. In all datasets for the development and validations, fold errors between predicted and observed pharmacokinetic parameters were within the two-fold error criteria. As a result, the PBPK model was appropriately established and properly described the pharmacokinetics of meloxicam in different CYP2C9 genotypes. This study is expected to contribute to reducing the risk of adverse events of meloxicam through optimization of meloxicam dosing in different CYP2C9 genotypes.




Similar content being viewed by others
References
Ahmad M, Murtaza G, Akhtar N, Siddique F, Khan SA (2011) Bioequivalence study of two brands of meloxicam tablets in healthy human Pakistani male subjects. Acta Pol Pharm 68:115–119
Ahmed M, Khanna D, Furst DE (2005) Meloxicam in rheumatoid arthritis. Expert Opin Drug Metab Toxicol 1(4):739–751. https://doi.org/10.1517/17425255.1.4.739
Aoyama T, Ishida Y, Kaneko M, Miyamoto A, Saito Y, Tohkin M, Kawai S, Matsumoto Y (2017) Pharmacokinetics and pharmacodynamics of meloxicam in East Asian populations: the role of ethnicity on drug response. CPT: Pharmacometrics Sys Pharmacol 6(12):823–832. https://doi.org/10.1002/psp4.12259
Awasthi SS, Kumar TG, Manisha P, Preeti Y, Kumar SS (2011) Development of meloxicam formulations utilizing ternary complexation for solubility enhancement. Pak J Pharm Sci 24(4):533–538
Bae JW, Kim MJ, Jang CG, Lee SY (2007) Determination of meloxicam in human plasma using a HPLC method with UV detection and its application to a pharmacokinetic study. J Chromatogr B 859(1):69–73. https://doi.org/10.1016/j.jchromb.2007.09.004
Bae JW, Choi CI, Jang CG, Lee SY (2011a) Effects of CYP2C9*1/*13 on the pharmacokinetics and pharmacodynamics of meloxicam. Br J Clin Pharmacol 71(4):550–555. https://doi.org/10.1111/j.1365-2125.2010.03853.x
Bae JW, Choi CI, Kim MJ, Oh DH, Keum SK, Park JI, Kim BH, Bang HK, Oh SG, Kang BS, Park HJ, Kim HD, Ha JH, Shin HJ, Kim YH, Na HS, Chung MW, Jang CG, Lee SY (2011b) Frequency of CYP2C9 alleles in Koreans and their effects on losartan pharmacokinetics. Acta Pharmacol Sin 32(10):1303–1308. https://doi.org/10.1038/aps.2011.100
Bae JW, Choi CI, Lee HI, Lee YJ, Jang CG, Lee SY (2012) Effects of CYP2C9*1/*3 and *1/*13 on the pharmacokinetics of losartan and its active metabolite E-3174. Int J Clin Pharmacol Ther 50(9):683–689. https://doi.org/10.5414/cp201467
Bae JW, Oh KY, Yoon SJ, Shin HB, Jung EH, Cho CK, Lim CW, Kang P, Choi CI, Jang CG, Lee SY, Lee YJ (2020) Effects of CYP2D6 genetic polymorphism on the pharmacokinetics of metoclopramide. Arch Pharm Res 43(11):1207–1213. https://doi.org/10.1007/s12272-020-01293-4
Barnette DA, Schleiff MA, Datta A, Flynn N, Swamidass SJ, Miller GP (2021) Meloxicam methyl group determines enzyme specificity for thiazole bioactivation compared to sudoxicam. Toxicol Lett 338:10–20. https://doi.org/10.1016/j.toxlet.2020.11.015
Boulton-Jones J, Geddes C, Heinzel G, Türck D, Nehmiz G, Bevis P (1997) Meloxicam pharmacokinetics in renal impairment. Br J Clin Pharmacol 43(1):35–40. https://doi.org/10.1111/j.1365-2125.1997.tb00030.x
Burgos-Vargas R, Foeldvari I, Thon A, Linke R, Tuerck D (2004) Pharmacokinetics of meloxicam in patients with juvenile rheumatoid arthritis. J Clin Pharmacol 44(8):866–872. https://doi.org/10.1177/0091270004267589
Busch U, Heinzel G, Narjes H (1995) The effect of cholestyramine on the pharmacokinetics of meloxicam, a new non-steroidal anti-inflammatory drug (NSAID), in man. Eur J Clin Pharmacol 48(3–4):269–272. https://doi.org/10.1007/BF00198310
Busch U, Heinzel G, Narjes H, Nehmiz G (1996a) Interaction of meloxicam with cimetidine, Maalox, or aspirin. J Clin Pharmacol 36(1):79–84. https://doi.org/10.1002/j.1552-4604.1996.tb04155.x
Busch U, Heinzel G, Narjes H, Nehmiz G, Türck D, Krimmer J, Rösch W (1996b) Pharmacokinetics of meloxicam in patients with hepatic cirrhosis in comparison with healthy volunteers. Clin Drug Invest 11(2):97–107. https://doi.org/10.2165/00044011-199611020-00005
Byeon JY, Lee CM, Lee YJ, Kim YH, Kim SH, Jung EH, Chae WK, Lee YJ, Jang CG, Lee SY (2019) Influence of CYP2D6 genetic polymorphism on pharmacokinetics of active moiety of tolterodine. Arch Pharm Res 42(2):182–190. https://doi.org/10.1007/s12272-018-1099-y
Céspedes-Garro C, Fricke-Galindo I, Naranjo ME, Rodrigues-Soares F, Fariñas H, de Andrés F, López-López M, Peñas-Lledó EM, LLerena A (2015) Worldwide interethnic variability and geographical distribution of CYP2C9 genotypes and phenotypes. Expert Opin Drug Metab Toxicol 11(12):1893–1905. https://doi.org/10.1517/17425255.2015.1111871
Chaubal G, Borkar VV, Shetty G, Chattopadhyay S, Bahure U, Badhe R, Udare A, Shah S, Gupte P, Shukla A, Rela M, Mohanka R (2016) Estimation of liver volume in the western Indian population. Indian J Gastroenterol 35(4):274–279. https://doi.org/10.1007/s12664-016-0662-z
Chesne C, Guyomard C, Guillouzo A, Schmid J, Ludwig E, Sauter T (1998) Metabolism of Meloxicam in human liver involves cytochromes P4502C9 and 3A4. Xenobiotica 28(1):1–13. https://doi.org/10.1080/004982598239704
Choi CI, Kim MJ, Jang CG, Park YS, Bae JW, Lee SY (2011) Effects of the CYP2C9*1/*13 genotype on the pharmacokinetics of lornoxicam. Basic Clin Pharmacol Toxicol 109(6):476–480. https://doi.org/10.1111/j.1742-7843.2011.00751.x
Choi CI, Kim MJ, Chung EK, Lee HI, Jang CG, Bae JW, Lee SY (2012) CYP2C9*3 and *13 alleles significantly affect the pharmacokinetics of irbesartan in healthy Korean subjects. Eur J Clin Pharmacol 68(2):149–154. https://doi.org/10.1007/s00228-011-1098-0
Carrasco-Portugal Mdel C, Aguilar-Carrasco JC, Luján M, Reyes-García G, Medina-Santillán R, Flores-Murrieta FJ (2005) Further evidence for interethnic differences in the oral pharmacokinetics of meloxicam. Clin Drug Investig 25(5):307–313. https://doi.org/10.2165/00044011-200525050-00003
Del Tacca M, Pasqualetti G, Gori G, Pepe P, Di Paolo A, Lastella M, De Negri F, Blandizzi C (2013) Comparative pharmacokinetic and pharmacodynamic evaluation of branded and generic formulations of meloxicam in healthy male volunteers. Ther Clin Risk Manag 9:303–311. https://doi.org/10.2147/TCRM.S39024
Duan P, Zhao P, Zhang L (2017) Physiologically based pharmacokinetic (PBPK) modeling of Pitavastatin and atorvastatin to predict drug-drug interactions (DDIs). Eur J Drug Metab Pharmacokinet 42(4):689–705. https://doi.org/10.1007/s13318-016-0383-9
Engelhardt G (1996) Pharmacology of meloxicam, a new non-steroidal anti-inflammatory drug with an improved safety profile through preferential inhibition of COX-2. Br J Rheumatol 35(Suppl 1):4–12. https://doi.org/10.1093/rheumatology/35.suppl_1.4
Gambero A, Becker TL, Zago AS, de Oliveira AF, Pedrazzoli J Jr (2005) Comparative study of anti-inflammatory and ulcerogenic activities of different cyclo-oxygenase inhibitors. Inflammopharmacology 13(5–6):441–454. https://doi.org/10.1163/156856005774649377
Gong J, Iacono L, Iyer RA, Humphreys WG, Zheng M (2018) Physiologically-based pharmacokinetic modelling of a CYP2C19 substrate, BMS-823778, utilizing pharmacogenetic data. Br J Clin Pharmacol 84(6):1335–1345. https://doi.org/10.1111/bcp.13565
Gschwend MH, Erenmemişoğlu A, Martin W, Tamur U, Kanzik I, Hincal AA (2007) Pharmacokinetic and bioequivalence study of meloxicam tablets in healthy male subjects. Arzneimittelforschung 57(05):264–268. https://doi.org/10.1055/s-0031-1296616
Hanft G, Türck D, Scheuerer S, Sigmund R (2001) Meloxicam oral suspension: a treatment alternative to solid meloxicam formulations. Inflamm Res 50(Suppl 1):S35-37. https://doi.org/10.1007/PL00000219
Hasan S, Shoaib MH, Hassan F, Rehman I (2009) Bioequivalence studies of two brands of meloxicam tablets in healthy Pakistani volunteers. Pak J Pharm Sci 22:199–204
Hashimoto T, Sugawara Y, Tamura S, Hasegawa K, Kishi Y, Kokudo N, Makuuchi M (2006) Estimation of standard liver volume in Japanese living liver donors. J Gastroenterol Hepatol 21(11):1710–1713. https://doi.org/10.1111/j.1440-1746.2006.04433.x
Hasunuma T, Tohkin M, Kaniwa N, Jang IJ, Yimin C, Kaneko M, Saito Y, Takeuchi M, Watanabe H, Yamazoe Y, Uyama Y, Kawai S (2016) Absence of ethnic differences in the pharmacokinetics of moxifloxacin, simvastatin, and meloxicam among three East Asian populations and Caucasians. Br J Clin Pharmacol 81(6):1078–1090. https://doi.org/10.1111/bcp.12884
Hatthapornsawan S, Sirivatanauksorn Y, Limsrichamrern S, Waiyawuth W (2004) Standard liver volume in Thai population. The Thai Journal of Surgery 25(3):84–86
Herden U, Wischhusen F, Heinemann A, Ganschow R, Grabhorn E, Vettorazzi E, Nashan B, Fischer L (2013) A formula to calculate the standard liver volume in children and its application in pediatric liver transplantation. Transpl Int 26(12):1217–1224. https://doi.org/10.1111/tri.12198
Hindmarsh AC, Reynolds DR, Serban R, Woodward CS, Gardner, DJ, Cohen SD. (2018) Open systems pharmacology suite manual,version 7.4.
Hynninen VV, Olkkola KT, Bertilsson L, Kurkinen KJ, Korhonen T, Neuvonen PJ, Laineet K (2009) Voriconazole increases while itraconazole decreases plasma meloxicam concentrations. Antimicrob Agents Chemother 53(2):587–592. https://doi.org/10.1128/AAC.00530-08
Boehringer Ingelheim (2012) Mobic® (meloxicam) prescribing information. In: Inc. BIP (ed)https://www.accessdata.fda.gov/drugsatfda_docs/label/2012/020938s022lbl.pdf. Ridgefield, USA
Jones H, Rowland-Yeo K (2013) Basic concepts in physiologically based pharmacokinetic modeling in drug discovery and development. CPT Pharmacometrics Syst Pharmacol 2(8):e63. https://doi.org/10.1038/psp.2013.41
Jung EH, Lee CM, Byeon JY, Shin HB, Oh KY, Cho CK, Lim CW, Jang CG, Lee SY, Lee YJ (2020a) Relationship between plasma exposure of zolpidem and CYP2D6 genotype in healthy Korean subjects. Arch Pharm Res 43(9):976–981. https://doi.org/10.1007/s12272-020-01250-1
Jung EH, Lee YJ, Kim DH, Kang P, Lim CW, Cho CK, Jang CG, Lee SY, Bae JW (2020b) Effects of paroxetine on the pharmacokinetics of atomoxetine and its metabolites in different CYP2D6 genotypes. Arch Pharm Res 43(12):1356–1363. https://doi.org/10.1007/s12272-020-01300-8
Kim SH, Kim DH, Byeon JY, Kim YH, Kim DH, Lim HJ, Lee CM, Whang SS, Choi CI, Bae JW, Lee YJ, Jang CG, Lee SY (2017) Effects of CYP2C9 genetic polymorphisms on the pharmacokinetics of celecoxib and its carboxylic acid metabolite. Arch Pharm Res 40(3):382–390. https://doi.org/10.1007/s12272-016-0861-2
Kim SH, Byeon JY, Kim YH, Lee CM, Lee YJ, Jang CG, Lee SY (2018) Physiologically based pharmacokinetic modelling of atomoxetine with regard to CYP2D6 genotypes. Sci Rep 8(1):12405. https://doi.org/10.1038/s41598-018-30841-8
Kim YH, Kang P, Cho CK, Jung EH, Park HJ, Lee YJ, Bae JW, Jang CG, Lee SY (2021) Physiologically based pharmacokinetic (PBPK) modeling for prediction of celecoxib pharmacokinetics according to CYP2C9 genetic polymorphism. Arch Pharm Res 44(7):713-724. https://doi.org/10.1007/s12272-021-01346-2
Kneller LA, Abad-Santos F, Hempel G (2020) Physiologically based pharmacokinetic modelling to describe the pharmacokinetics of risperidone and 9-hydroxyrisperidone according to cytochrome P450 2D6 phenotypes. Clin Pharmacokinet 59(1):51–65. https://doi.org/10.1007/s40262-019-00793-x
Kneller LA, Zubiaur P, Koller D, Abad-Santos F, Hempel G (2021) Influence of CYP2D6 phenotypes on the pharmacokinetics of Aripiprazole and Dehydro-Aripiprazole using a physiologically based pharmacokinetic approach. Clin Pharmacokinet, in press. https://doi.org/10.1007/s40262-021-01041-x
Kromrey M, Ittermann T, vWahsen C, Plodeck V, Seppelt D, Hoffmann RT, Heiss P, Kühn JP (2018) Reference values of liver volume in Caucasian population and factors influencing liver size. Eur J Radiol 106:32–37. https://doi.org/10.1016/j.ejrad.2018.07.005
Lee HI, Bae JW, Choi CI, Lee YJ, Byeon JY, Jang CG, Lee SY (2014) Strongly increased exposure of meloxicam in CYP2C9*3/*3 individuals. Pharmacogenet Genomics 24(2):113–117. https://doi.org/10.1097/fpc.0000000000000025
Lee YJ, Byeon JY, Kim YH, Kim SH, Choi CI, Bae JW, Sohn UD, Jang CG, Lee J, Lee SY (2015) Effects of CYP2C9*1/*3 genotype on the pharmacokinetics of flurbiprofen in Korean subjects. Arch Pharm Res 38(6):1232–1237. https://doi.org/10.1007/s12272-015-0580-0
Lee CM, Jung EH, Byeon JY, Kim SH, Jang CG, Lee YJ, Lee SY (2019) Effects of steady-state clarithromycin on the pharmacokinetics of zolpidem in healthy subjects. Arch Pharm Res 42(12):1101–1106. https://doi.org/10.1007/s12272-019-01201-5
Lee J, Yang Y, Zhang X, Fan J, Grimstein M, Zhu H, Wang Y (2021) Usage of in vitro metabolism data for drug-drug interaction in physiologically based pharmacokinetic (PBPK) analyses submissions to the US food and drug administration. J Clin Pharmacol 61(6):782–788. https://doi.org/10.1002/jcph.1819
Liu R, Gong C, Tao L, Yang W, Zheng X, Ma P, Ding L (2015) Influence of genetic polymorphisms on the pharmacokinetics of celecoxib and its two main metabolites in healthy Chinese subjects. Eur J Pharm Sci 79:13–19. https://doi.org/10.1016/j.ejps.2015.09.005
Marcelín-Jiménez G, Hernández JA, Angeles AP, Contreras L, García A, Hinojosa M, Morales M, Rivera L, Martínez-Rossier L, Fernández A (2005) Bioequivalence evaluation of two brands of meloxicam tablets (promotion® and mobicox®): pharmacokinetics in a healthy female Mexican population. Biopharm Drug Dispos 26(5):167–171. https://doi.org/10.1002/bdd.446
Martin L, Hutchens M, Hawkins C, Radnov A (2017) How much do clinical trials cost? Nat Rev Drug Discov 16(6):381–382. https://doi.org/10.1038/nrd.2017.70
Medvedovici A, Albu F, Georgita C, Mircioiu C, David V (2005) A non-extracting procedure for the determination of meloxicam in plasma samples by HPLC-diode array detection. Arzneimittelforschung 55(6):326–331. https://doi.org/10.1055/s-0031-1296867
Mishra DN, Vijaya Kumar SG (2006) Investigations on analgesic, anti-inflammatory and ulcerogenic potential of meloxicam solid dispersion prepared with skimmed milk. Yakugaku Zasshi 126(7):495–498. https://doi.org/10.1248/yakushi.126.495
Moraes de Oliveira RT, Dias ARN, Vieira WB, Falcão ASC, Falcão LFM, Quaresma JAS (2020) A protocol of hepatic volume measurement using magnetic resonance imaging in individuals from the Eastern Brazilian Amazon population. PLoS ONE 15(3):e0229525. https://doi.org/10.1371/journal.pone.0229525
Mosteller R (1987) Simplified calculation of body-surface area. N Engl J Med 317:1098. https://doi.org/10.1056/NEJM198710223171717
Nishimura M, Naito S (2005) Tissue-specific mRNA expression profiles of human ATP-binding cassette and solute carrier transporter superfamilies. Drug Metab Pharmacokinet 20(6):452–477. https://doi.org/10.2133/dmpk.20.452
Nishimura M, Naito S (2006) Tissue-specific mRNA expression profiles of human phase I metabolizing enzymes except for cytochrome P450 and phase II metabolizing enzymes. Drug Metab Pharmacokinet 21(5):357–374. https://doi.org/10.2133/dmpk.21.357
Nishimura M, Yaguti H, Yoshitsugu H, Naito S, Satoh T (2003) Tissue distribution of mrna expression of human cytochrome P450 isoforms assessedby high-sensitivity real-time reverse transcription PCR. Yakugaku Zasshi 123(5):369–375. https://doi.org/10.1248/yakushi.123.369
Radicioni M, Connolly S, Stroppolo F, Granata G, Loprete L, Leuratti C (2013) Bioequivalence study of a novel orodispersible tablet of meloxicam in a porous matrix after single-dose administration in healthy volunteers. Int J Clin Pharmacol Ther 51(3):234–243. https://doi.org/10.5414/cp201781
Rigato HM, Mendes GD, Borges NC, Moreno R (2006) Meloxicam determination in human plasma by high-performance liquid chromatography coupled with tandem mass spectrometry (LC-MS-MS) in Brazilian bioequivalence studies. Int J Clin Pharmacol Ther 44(10):489–498. https://doi.org/10.5414/cpp44489
Ross KA, Bigham AW, Edwards M, Gozdzik A, Suarez-Kurtz G, Parra EJ (2010) Worldwide allele frequency distribution of four polymorphisms associated with warfarin dose requirements. J Hum Genet 55(9):582–589. https://doi.org/10.1038/jhg.2010.73
Sager JE, Yu J, Ragueneau-Majlessi I, Isoherranen N (2015) Physiologically based pharmacokinetic (PBPK) modeling and simulation approaches: a systematic review of published models, applications, and model verification. Drug Metab Dispos 43(11):1823–1837. https://doi.org/10.1124/dmd.115.065920
Schlender J-F, Teutonico D, Coboeken K, Schnizler K, Eissing T, Willmann S, Jaehde U, Stass H (2018) A physiologically-based pharmacokinetic model to describe ciprofloxacin pharmacokinetics over the entire span of life. Clin Pharmacokinet 57(12):1613–1634. https://doi.org/10.1007/s40262-018-0661-6
Schmitt W (2008) General approach for the calculation of tissue to plasma partition coefficients. Toxicol in Vitro 22(2):457–467. https://doi.org/10.1016/j.tiv.2007.09.010
Schoenfeld P (1999) Gastrointestinal safety profile of meloxicam: a meta-analysis and systematic review of randomized controlled trials. Am J Med 107(6A):48S-54S. https://doi.org/10.1016/s0002-9343(99)00367-8
Shi ZR, Yan LN, Li B, Wen TF (2009) Evaluation of standard liver volume formulae for Chinese adults. World J Gastroenterol 15(32):4062–4066. https://doi.org/10.3748/wjg.15.4062
Shin HB, Jung EH, Kang P, Lim CW, Oh KY, Cho CK, Lee YJ, Choi CI, Jang CG, Lee SY, Bae JW (2020) ABCB1 c.2677G>T/c.3435C>T diplotype increases the early-phase oral absorption of losartan. Arch Pharm Res 43(11):1187–1196. https://doi.org/10.1007/s12272-020-01294-3
Theken KN, Lee CR, Gong L, Caudle KE, Formea CM, Gaedigk A, Klein TE, Agúndez JAG, Grosser T (2020) Clinical Pharmacogenetics implementation consortium guideline (CPIC) for CYP2C9 and nonsteroidal anti-inflammatory drugs. Clin Pharmacol Ther 108(2):191–200. https://doi.org/10.1002/cpt.1830
Thelen K, Coboeken K, Willmann S, Burghaus R, Dressman JB, Lippert J (2011) Evolution of a detailed physiological model to simulate the gastrointestinal transit and absorption process in humans, part 1: oral solutions. J Pharm Sci 100(12):5324–5345. https://doi.org/10.1002/jps.22726
Thelen K, Coboeken K, Willmann S, Dressman JB, Lippert J (2012) Evolution of a detailed physiological model to simulate the gastrointestinal transit and absorption process in humans, part II: extension to describe performance of solid dosage forms. J Pharm Sci 101(3):1267–1280. https://doi.org/10.1002/jps.22825
Thummati P, Kumsorn B, Rojanasthien N (2004) Bioequivalence study of the generic meloxicam (Melox®) compared with the innovator Mobic®. J Basic App Pharmacol 26(3):189–199
Türck D, Busch U, Heinzel G, Narjes H, Nehmiz G (1995) Effect of food on the pharmacokinetics of meloxicam after oral administration. Clin Drug Investig 9(5):270–276. https://doi.org/10.2165/00044011-199509050-00004
Türck D, Schwarz A, Höffler D, Narjes H, Nehmiz G, Heinzel G (1996) Pharmacokinetics of meloxicam in patients with end-stage renal failure on haemodialysis: a comparison with healthy volunteers. Eur J Clin Pharmacol 51(3–4):309–313. https://doi.org/10.1007/s002280050203
Türck D, Busch U, Heinzel G, Narjes H (1997) Clinical Pharmacokinetics of Meloxicam. Arzneimittel-Forschung 47(3):253–258
Um EH, Hwang S, Song G-W, Jung DH, Ahn CS, Kim KH, Moon DB, Park GC, Lee SG (2015) Calculation of standard liver volume in Korean adults with analysis of confounding variables. Korean J Hepatobiliary Pancreat Surg 19(4):133–138. https://doi.org/10.14701/kjhbps.2015.19.4.133
Williams BS (2018) Nonopioid analgesics: Nonsteroidal antiinflammatory drugs, cyclooxygenase-2 inhibitors, and acetaminophen. Essentials of Pain Medicine. Elsevier, Amsterdam, pp 457–468
Xu M, Zheng L, Zeng J, Xu W, Jiang X, Wang L (2021) Physiologically based pharmacokinetic modeling of tramadol to inform dose adjustment and drug-drug interactions according to CYP2D6 phenotypes. Pharmacotherapy 41(3):277–290. https://doi.org/10.1002/phar.2494
Yoshizumi T, Gondolesi GE, Bodian CA, Jeon H, Schwartz ME, Fishbein TM, Miller CM, Emre S (2003) A simple new formula to assess liver weight. Transplant Proc 35(4):1415–1420. https://doi.org/10.1016/s0041-1345(03)00482-2
Yu J, Wang Y, Wu Y, Lin S, Hao R, Fang L, Zhu J, Zhao D, Tong S, Si Y, Ye T, Wu Z, Huang H, Zhou F, Wang Y (2021) Pharmacokinetics of Meloxicam tablets in healthy chinese adults in the fasting and fed states: A single-site, single-dose, randomized, open, 2-period, 2-sequence, crossover bioequivalence study. Clin Pharmacol Drug Dev, in press. https://doi.org/10.1002/cpdd.965
Zhang M, Yang Y, Zhao G, Di X, Xu L, Jiang N, Xu J, Xu X (2014) Effect of CYP2C9* 3 mutant variants on meloxicam pharmacokinetics in a healthy Chinese population. Genet Mol Res 13(1):831–837. https://doi.org/10.4238/2014.February.13.1
Zhuang X, Lu C (2016) PBPK modeling and simulation in drug research and development. Acta Pharm Sin B 6(5):430–440. https://doi.org/10.1016/j.apsb.2016.04.004
Acknowledgements
This research was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT, and Future Planning (NRF-2019R1A2C1004582)
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cho, C., Park, HJ., Kang, P. et al. Physiologically based pharmacokinetic (PBPK) modeling of meloxicam in different CYP2C9 genotypes. Arch. Pharm. Res. 44, 1076–1090 (2021). https://doi.org/10.1007/s12272-021-01361-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12272-021-01361-3