Abstract
Diabetic cardiomyopathy (DCM) is one of the major causes of heart failure in diabetic patients. However, the pathogenesis of diabetic cardiomyopathy has not been fully elucidated. Diagnosis and therapeutic strategy of DCM is still challenging. Various non-coding RNAs (ncRNA) are implicated in the onset and progression of DCM. Interestingly, ncRNAs not only are regulators intracellularly, but also can exist and function in extracellular space. Recent evidences have demonstrated that extracellular ncRNAs play emerging roles in both intracardiac and inter-organ communication during the pathogenesis of DCM; thus, extracellular ncRNAs are attractive diagnostic biomarkers and potential therapeutic targets for DCM. This article will review the current knowledge of the roles of extracellular ncRNAs in DCM, especially focusing on their physio-pathological properties and perspectives of potential clinical translation for biomarkers and therapies.
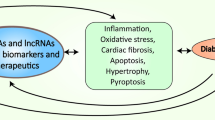
Graphical abstract
Recent evidences have demonstrated that extracellular ncRNA play emerging roles in both intracardiac and inter-organ communication involved in the pathogenesis of diabetic cardiomyopathy (DCM), thus shown as attractive diagnostic biomarkers and potential therapeutics for DCM. In the current review, we first summarize the progress regarding the paracrine role of extracellular ncRNA in DCM. miRNAs and circRNAs have been shown to mediate the communication among cardiomyocytes, endothelial cells, and vascular smooth muscle cells in the diabetic heart. Subsequently, we systematically describe that extracellular ncRNAs contribute to the crosstalk between the heart and other organs in the context of diabetes. Researches have indicated that miRNAs acted as hepatokines and adipokines to mediates the injure effect of distal organs on hearts. As for clinical application, extracellular ncRNAs are promising biomarker and have therapeutic potential. (Created with BioRender.com)



Similar content being viewed by others
Abbreviations
- DCM:
-
Diabetic cardiomyopathy
- ncRNA:
-
Non-coding RNAs
- DM:
-
Diabetes mellitus
- HF:
-
Heart failure
- CHD:
-
Coronary heart disease
- DPP-4:
-
Dipeptidylpeptidase-4
- GLP-1:
-
Glucagon-like peptide-1
- SGLT2:
-
Sodium-dependent glucose transporters 2
- lncRNA:
-
Long ncRNA
- miRNA:
-
MicroRNA
- siRNA:
-
Short interfering RNA
- snoRNA:
-
Small nuclear RNA
- piRNA:
-
Piwi-interacting RNA
- ECM:
-
Extracellular matrix
- EVs:
-
Extracellular vesicles
- MVB:
-
Multi-vesicular body
- T2D:
-
Type 2 diabetes
- MCECs:
-
Mouse cardiac endothelial cells
- CMR:
-
Cardiac magnetic resonance
References
H. Wang, N. Li, T. Chivese, M. Werfalli, H. Sun, L. Yuen, C. Ambrosius Hoegfeldt, C. Elise Powe, J. Immanuel, S. Karuranga, H. Divakar, N. Levitt, C. Li, D. Simmons, X. Yang, IDF Diabetes atlas: Global and regional estimate of gestational diabetes mellitus prevalence for 2019-2021 by International Association of Diabetes in Pregnancy Study Group’s Criteria, Diabetes Research Clinical Practice (2021) 183:109050.
Tan, Y., Zhang, Z., Zheng, C., Wintergerst, K. A., Keller, B. B., & Cai, L. (2020). Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nature Reviews Cardiology, 17(9), 585–607.
Jia, G., Hill, M. A., & Sowers, J. R. (2018). Diabetic cardiomyopathy: An update of mechanisms contributing to this clinical entity. Circulation Research, 122(4), 624–638.
Varma, U., Koutsifeli, P., Benson, V. L., Mellor, K. M., & Delbridge, L. M. D. (2018). Molecular mechanisms of cardiac pathology in diabetes - Experimental insights. Biochimica Biophysica Acta Molecular Basis Disease, 1864(5 Pt B), 1949–1959.
Beermann, J., Piccoli, M.-T., Viereck, J., & Thum, T. (2016). Non-coding RNAs in development and disease: Background, mechanisms, and therapeutic approaches. Physiol Rev, 96(4), 1297–1325.
Matsui, M., & Corey, D. R. (2017). Non-coding RNAs as drug targets. Nature Reviews Drug Discovery, 16(3), 167–179.
Fasolo, F., Di Gregoli, K., Maegdefessel, L., & Johnson, J. L. (2019). Non-coding RNAs in cardiovascular cell biology and atherosclerosis. Cardiovascular Research, 115(12), 1732–1756.
Li, H., Fan, J., Chen, C., & Wang, D. W. (2020). Subcellular microRNAs in diabetic cardiomyopathy. Annals Translational Medicine, 8(23), 1602.
Jakubik, D., Fitas, A., Eyileten, C., Jarosz-Popek, J., Nowak, A., Czajka, P., Wicik, Z., Sourij, H., Siller-Matula, J. M., De Rosa, S., & Postula, M. (2021). MicroRNAs and long non-coding RNAs in the pathophysiological processes of diabetic cardiomyopathy: emerging biomarkers and potential therapeutics. Cardiovascular Diabetology, 20(1), 55.
Sato-Kuwabara, Y., Melo, S. A., Soares, F. A., & Calin, G. A. (2015). The fusion of two worlds: non-coding RNAs and extracellular vesicles--diagnostic and therapeutic implications (Review). International Journal of Oncology, 46(1), 17–27.
Videira, R. F., & da Costa Martins, P. A. (2020). Non-coding RNAs in cardiac intercellular communication. Front Physiology, 11, 738.
Hu, W., Liu, C., Bi, Z.-Y., Zhou, Q., Zhang, H., Li, L.-L., Zhang, J., Zhu, W., Song, Y.-Y.-Y., Zhang, F., Yang, H.-M., Bi, Y.-Y., He, Q.-Q., Tan, G.-J., Sun, C.-C., & Li, D.-J. (2020). Comprehensive landscape of extracellular vesicle-derived RNAs in cancer initiation, progression, metastasis and cancer immunology. Molecular Cancer, 19(1), 102.
Anfossi, S., Babayan, A., Pantel, K., & Calin, G. A. (2018). Clinical utility of circulating non-coding RNAs - An update. Nature Reviews Clinical Oncology, 15(9), 541–563.
Xiao, Y., Zheng, L., Zou, X., Wang, J., Zhong, J., & Zhong, T. (2019). Extracellular vesicles in type 2 diabetes mellitus: Key roles in pathogenesis, complications, and therapy. Journal of Extracellular Vesicles, 8(1), 1625677.
Vickers, K. C., Palmisano, B. T., Shoucri, B. M., Shamburek, R. D., & Remaley, A. T. (2011). MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nature Cell Biology, 13(4), 423–433.
Turchinovich, A., Weiz, L., Langheinz, A., & Burwinkel, B. (2011). Characterization of extracellular circulating microRNA. Nucleic Acids Research, 39(16), 7223–7233.
Colombo, M., Raposo, G., & Théry, C. (2014). Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annual Review of Cell Development Biology, 30, 255–289.
Mulcahy, L. A., Pink, R. C., & Carter, D. R. F. (2014). Routes and mechanisms of extracellular vesicle uptake. Journal of Extracellular Vesicles, 4, 3.
Joshi, B. S., de Beer, M. A., Giepmans, B. N. G., & Zuhorn, I. S. (2020). Endocytosis of extracellular vesicles and release of their cargo from endosomes. ACS Nano, 14(4), 4444–4455.
Han, C., Yang, J., Sun, J., Qin, G. (2021). Extracellular vesicles in cardiovascular disease: Biological functions and therapeutic implications. Pharmacology & Therapeutics, 108025.
Liang, Y., Lehrich, B. M., Zheng, S., & Lu, M. (2021). Emerging methods in biomarker identification for extracellular vesicle-based liquid biopsy. Journal of Extracellular Vesicles, 10(7), e12090.
Kang, M., Jordan, V., Blenkiron, C., & Chamley, L. W. (2021). Biodistribution of extracellular vesicles following administration into animals: A systematic review. Journal of Extracellular Vesicles, 10(8), e12085.
Catalano, M., & O'Driscoll, L. (2020). Inhibiting extracellular vesicles formation and release: a review of EV inhibitors. Journal of Extracellular Vesicles, 9(1), 1703244.
Nguyen, V. V. T., Witwer, K. W., Verhaar, M. C., Strunk, D., & van Balkom, B. W. M. (2020). Functional assays to assess the therapeutic potential of extracellular vesicles. Journal of Extracellular Vesicles, 10(1), e12033.
Yin, Y., Chen, H., Wang, Y., Zhang, L., & Wang, X. (2021). Roles of extracellular vesicles in the aging microenvironment and age-related diseases. Journal of Extracellular Vesicles, 10(12), e12154.
Pinto, A. R., Ilinykh, A., Ivey, M. J., Kuwabara, J. T., D'Antoni, M. L., Debuque, R., Chandran, A., Wang, L., Arora, K., Rosenthal, N. A., & Tallquist, M. D. (2016). Revisiting cardiac cellular composition. Circulation Research, 118(3), 400–409.
Banerjee, I., Fuseler, J. W., Price, R. L., Borg, T. K., & Baudino, T. A. (2007). Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. American Journal of Physiology-Heart and Circulatory Physiology, 293(3), H1883–H1891.
Shome, J. S., Perera, D., Plein, S., & Chiribiri, A. (2017). Current perspectives in coronary microvascular dysfunction. Microcirculation, 24(1).
Lopez, J. J., Laham, R. J., Carrozza, J. P., Tofukuji, M., Sellke, F. W., Bunting, S., & Simons, M. (1997). Hemodynamic effects of intracoronary VEGF delivery: Evidence of tachyphylaxis and NO dependence of response. American Journal of Physiology, 273(3 Pt 2), H1317–H1323.
Widyantoro, B., Emoto, N., Nakayama, K., Anggrahini, D. W., Adiarto, S., Iwasa, N., Yagi, K., Miyagawa, K., Rikitake, Y., Suzuki, T., Kisanuki, Y. Y., Yanagisawa, M., & Hirata, K.-I. (2010). Endothelial cell-derived endothelin-1 promotes cardiac fibrosis in diabetic hearts through stimulation of endothelial-to-mesenchymal transition. Circulation, 121(22), 2407–2418.
Sluijter, J. P., Verhage, V., Deddens, J. C., van den Akker, F., & Doevendans, P. A. (2014). Microvesicles and exosomes for intracardiac communication. Cardiovascular Research, 102(2), 302–311.
Wang, X., Huang, W., Liu, G., Cai, W., Millard, R. W., Wang, Y., Chang, J., Peng, T., & Fan, G. C. (2014). Cardiomyocytes mediate anti-angiogenesis in type 2 diabetic rats through the exosomal transfer of miR-320 into endothelial cells. Journal of Molecular and Cellular Cardiology, 74, 139–150.
Garcia, N. A., Ontoria-Oviedo, I., González-King, H., Diez-Juan, A., & Sepúlveda, P. (2015). Glucose starvation in cardiomyocytes enhances exosome secretion and promotes angiogenesis in endothelial cells. PLoS One, 10(9), e0138849.
Chaturvedi, P., Kalani, A., Medina, I., Familtseva, A., & Tyagi, S. C. (2015). Cardiosome mediated regulation of MMP9 in diabetic heart: Role of mir29b and mir455 in exercise. Journal of Cellular and Molecular Medicine, 19(9), 2153–2161.
Wang, S., Zhan, J., Lin, X., Wang, Y., Wang, Y., & Liu, Y. (2020). CircRNA-0077930 from hyperglycaemia-stimulated vascular endothelial cell exosomes regulates senescence in vascular smooth muscle cells. Cell Biochemistry and Function, 38(8), 1056–1068.
Khalil, N. N., & McCain, M. L. (2021). Engineering the cellular microenvironment of post-infarct myocardium on a chip. Front Cardiovascular Medicine, 8, 709871.
Cecen, B., Karavasili, C., Nazir, M., Bhusal, A., Dogan, E., Shahriyari, F., Tamburaci, S., Buyukoz, M., Kozaci, L. D., & Miri, A. K. (2021). Multi-organs-on-chips for testing small-molecule drugs: Challenges and perspectives. Pharmaceutics, 13(10), 1657.
Severinsen, M. C. K., Pedersen, B. K. (2020). Muscle-organ crosstalk: The emerging roles of myokines. Endocrine Reviews, 41(4).
Meex, R. C. R., & Watt, M. J. (2017). Hepatokines: Linking nonalcoholic fatty liver disease and insulin resistance. Nature Reviews Endocrinology, 13(9), 509–520.
Fasshauer, M., & Blüher, M. (2015). Adipokines in health and disease. Trends in Pharmacological Sciences, 36(7), 461–470.
Li, C.-J., Fang, Q.-H., Liu, M.-L., & Lin, J.-N. (2020). Current understanding of the role of adipose-derived extracellular vesicles in metabolic homeostasis and diseases: Communication from the distance between cells/tissues. Theranostics, 10(16), 7422–7435.
Fang, X., Stroud, M. J., Ouyang, K., Fang, L., Zhang, J., Dalton, N. D., Gu, Y., Wu, T., Peterson, K. L., Huang, H.-D., Chen, J., & Wang, N. (2016). Adipocyte-specific loss of PPAR attenuates cardiac hypertrophy. JCI Insight, 1(16), e89908.
Gan, L., Xie, D., Liu, J., Bond Lau, W., Christopher, T. A., Lopez, B., Zhang, L., Gao, E., Koch, W., Ma, X.-L., & Wang, Y. (2020). Small extracellular microvesicles mediated pathological communications between dysfunctional adipocytes and cardiomyocytes as a novel mechanism exacerbating ischemia/reperfusion injury in diabetic mice. Circulation, 141(12), 968–983.
Wang, Y., Jin, P., Liu, J., & Xie, X. (2019). Exosomal microRNA-122 mediates obesity-related cardiomyopathy through suppressing mitochondrial ADP-ribosylation factor-like 2. Clinical Science (Lond), 133(17), 1871–1881.
Nie, H., Pan, Y., & Zhou, Y. (2018). Exosomal microRNA-194 causes cardiac injury and mitochondrial dysfunction in obese mice. Biochemical Biophysical Research Communications, 503(4), 3174–3179.
Li, F., Zhang, K., Xu, T., Du, W., Yu, B., Liu, Y., & Nie, H. (2019). Exosomal microRNA-29a mediates cardiac dysfunction and mitochondrial inactivity in obesity-related cardiomyopathy. Endocrine, 63(3), 480–488.
Xu, M.-Y., Ye, Z.-S., Song, X.-T., & Huang, R.-C. (2019). Differences in the cargos and functions of exosomes derived from six cardiac cell types: A systematic review. Stem Cell Research and Therapy, 10(1), 194.
Sun, L.-L., Duan, M.-J., Ma, J.-C., Xu, L., Mao, M., Biddyut, D., Wang, Q., Yang, C., Zhang, S., Xu, Y., Yang, L., Tian, Y., Liu, Y., Xia, S.-N., Li, K.-X., Jin, Z., Xiong, Q., & Ai, J. (2018). Myocardial infarction-induced hippocampal microtubule damage by cardiac originating microRNA-1 in mice. Journal of Molecular and Cellular Cardiology, 120, 12–27.
Paolillo, S., Marsico, F., Prastaro, M., Renga, F., Esposito, L., De Martino, F., Di Napoli, P., Esposito, I., Ambrosio, A., Ianniruberto, M., Mennella, R., Paolillo, R., & Gargiulo, P. (2019). Diabetic cardiomyopathy: Definition, diagnosis, and therapeutic implications. Heart Failure Clinics, 15(3), 341–347.
de Gonzalo-Calvo, D., van der Meer, R. W., Rijzewijk, L. J., Smit, J. W. A., Revuelta-Lopez, E., Nasarre, L., Escola-Gil, J. C., Lamb, H. J., & Llorente-Cortes, V. (2017). Serum microRNA-1 and microRNA-133a levels reflect myocardial steatosis in uncomplicated type 2 diabetes. Scientific Reports, 7(1), 47.
Tao, L., Huang, X., Xu, M., Qin, Z., Zhang, F., Hua, F., Jiang, X., & Wang, Y. (2020). Value of circulating miRNA-21 in the diagnosis of subclinical diabetic cardiomyopathy. Molecular and Cellular Endocrinology, 518, 110944.
Pofi, R., Giannetta, E., Galea, N., Francone, M., Campolo, F., Barbagallo, F., Gianfrilli, D., Venneri, M. A., Filardi, T., Cristini, C., Antonini, G., Badagliacca, R., Frati, G., Lenzi, A., Carbone, I., & Isidori, A. M. (2021). Diabetic cardiomiopathy progression is triggered by miR122-5p and involves extracellular matrix: A 5-year prospective study. JACC Cardiovasc Imaging, 14(6), 1130–1142.
Li, H., Fan, J., Zhao, Y., Zhang, X., Dai, B., Zhan, J., Yin, Z., Nie, X., Fu, X.-D., Chen, C., & Wang, D. W. (2019). Nuclear miR-320 mediates diabetes-induced cardiac dysfunction by activating transcription of fatty acid metabolic genes to cause lipotoxicity in the heart. Circulation Research, 125(12), 1106–1120.
Copier, C. U., León, L., Fernández, M., Contador, D., & Calligaris, S. D. (2017). Circulating miR-19b and miR-181b are potential biomarkers for diabetic cardiomyopathy. Scientific Reports, 7(1), 13514.
Pant, T., Dhanasekaran, A., Zhao, M., Thorp, E. B., Forbess, J. M., Bosnjak, Z. J., Benjamin, I. J., & Ge, Z.-D. (2021). Identification and analysis of circulating long non-coding RNAs with high significance in diabetic cardiomyopathy. Scientific Reports, 11(1), 2571.
Wang, X., He, Y., Mackowiak, B., & Gao, B. (2021). MicroRNAs as regulators, biomarkers and therapeutic targets in liver diseases. Gut, 70(4), 784–795.
Ling, H. (2016). Non-coding RNAs: Therapeutic strategies and delivery systems. Advances Experimental Medicine and Biology, 937, 229–237.
Huang, C.-K., Kafert-Kasting, S., & Thum, T. (2020). Preclinical and clinical development of noncoding RNA Therapeutics for cardiovascular disease. Circulation Research, 126(5), 663–678.
Funding
This work was supported by grants from the National Natural Science Foundation of China (nos. 81822002, 31771264, and 82100375). The funders had no role in the study design, data collection and analysis, manuscript preparation, or decision to publish.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Consent to Participate
No human studies were carried out by the authors for this article.
Conflict of Interest
The authors declare no competing interests.
Additional information
Associate Editor Junjie Xiao oversaw the review of this article
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Yin, Z., Chen, C. Biological Functions and Clinical Prospects of Extracellular Non-Coding RNAs in Diabetic Cardiomyopathy: an Updated Review. J. of Cardiovasc. Trans. Res. 15, 469–476 (2022). https://doi.org/10.1007/s12265-022-10217-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12265-022-10217-0