Abstract
A multi-year study was conducted in the eutrophic Lafayette River, a sub-tributary of the lower Chesapeake Bay during which uptake of inorganic and organic nitrogen (N) and C compounds was measured during multiple seasons and years when different dinoflagellate species were dominant. Seasonal dinoflagellate blooms included a variety of mixotrophic dinoflagellates including Heterocapsa triquetra in the late winter, Prorocentrum minimum in the spring, Akashiwo sanguinea in the early summer, and Scrippsiella trochoidea and Cochlodinium polykrikoides in late summer and fall. Results showed that no single N source fueled algal growth, rather rates of N and C uptake varied on seasonal and diurnal timescales, and within blooms as they initiated and developed. Rates of photosynthetic C uptake were low yielding low assimilation numbers during much of the study period and the ability to assimilate dissolved organic carbon augmented photosynthetic C uptake during bloom and non-bloom periods. The ability to use dissolved organic C during the day and night may allow mixotrophic bloom organisms a competitive advantage over co-occurring phytoplankton that are restricted to photoautotrophic growth, obtaining N and C during the day and in well-lit surface waters.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The number and magnitude of dinoflagellate blooms within the Chesapeake Bay region have increased over the last several decades (Li et al. 2015), and this is alarming because many of these dinoflagellate species are potentially harmful (Marshall et al. 2009). The initiation and expansion of harmful algal blooms have been linked to eutrophication (Anderson et al. 2002, 2008; Glibert et al. 2005; Heisler et al. 2008). Estuaries like the Chesapeake Bay receive large inorganic and organic nitrogen (N) and phosphorus (P) loads from adjacent coastal communities through point and non-point sources that fuel excess algal growth. While the bioavailability of inorganic nutrients is well-established, most of the bloom-forming species in the lower Chesapeake Bay can also take up dissolved organic nitrogen (DON) compounds (Berman and Bronk 2003; Mulholland and Lomas 2008). Recent genomic evidence has confirmed that many phytoplankton taxa are capable of taking up more components of the dissolved organic matter (DOM) pool than previously thought (Berg et al. 2008; Gobler et al. 2011; Mulholland and Lee 2009) and this may augment dissolved inorganic nitrogen (DIN) uptake.
The phytoplankton community in the lower Chesapeake Bay includes a variety of potentially bloom-forming dinoflagellates (e.g., Prorocentrum minimum, Akashiwo sanguinea, Heterocapsa triquetra, Heterocapsa rotundatum, Cochlodinium polykrikoides, and Gymnodinium spp.) (Marshall et al. 2003, 2005, 2009) that are known to be mixotrophic (Jeong et al. 2005a, b, 2015; Lewitus 2006; Burkholder et al. 2008). Mixotrophs are capable of photoautotrophic carbon (C) assimilation as well as heterotrophic C acquisition through grazing on co-occurring plankton (phagotrophy) and/or uptake of DOM (osmotrophy) (Graneli et al. 1999; Stoecker 1999; Burkholder et al. 2008; Hansen 2011; Jeong et al. 2015). Mixotrophic metabolisms were originally thought to be advantageous in oligotrophic environments where inorganic nutrients might be limiting (e.g., Tittel et al. 2003); however, metabolic flexibility is now known to be beneficial to organisms in eutrophic environments where light may limit photoautotrophic growth and N-rich organic compounds are abundant (Burkholder et al. 2008).
While N acquisition from organic compounds is now widely documented, uptake of associated C is not. Many algal species and groups are known to take up organic N compounds; these compounds can also provide C to support algal growth, as has been proposed for the brown tide pelagophyte, Aureococcus anophagefferens (Dzurica et al. 1989; Mulholland et al. 2002, 2009a). During advanced stages of monospecific algal blooms, cell densities are high over long periods, and this may result in C or light limitation of photosynthesis. Osmotrophy can provide C and other elements necessary for growth because uptake of DOM and grazing do not require light. We posit that osmotrophy could be advantageous for mixotrophic dinoflagellates during bloom initiation, when phytoplankton groups compete for diverse organic and inorganic N compounds and during mature blooms, when inorganic nutrients are depleted, light penetration may be limited, and particulate or dissolved organic N may be the dominant bioavailable N.
The Lafayette River, a sub-tributary of the lower Chesapeake Bay, has a diverse dinoflagellate assemblage, including many species that exert harmful effects either directly or indirectly, through ecosystem disruption and their contribution to oxygen depletion during bloom decay (Marshall 1968, 1995; Mulholland et al. 2009a, b; Egerton et al. 2014; Morse et al. 2013, 2014). Species known to bloom there include a variety of mixotrophic dinoflagellates: H. triquetra in the late winter, P. minimum in the spring, A. sanguinea in the early summer, Gymnodinium sp. throughout the summer, and Scrippsiella trochoidea and Cochlodinium sp. later in the summer and fall (Fig. 1). The Lafayette and adjacent Elizabeth Rivers (Fig. 2) also appear to be an initiation site for blooms that spread and are transported into the lower Chesapeake Bay (Morse et al. 2011, 2013). The system is tidally dominated and has a long residence time (1 to 4 months; White 1972) and this is thought to contribute to the incubation of algal populations during bloom initiation (Morse et al. 2013). Freshwater inputs are limited to precipitation, shoreline drainage, groundwater inputs, and controlled flow into the southern branch of the Elizabeth River (Kim et al. 2002; Charette and Buessler 2004).
Box and whisker plot showing the occurrence of dinoflagellate blooms (> 1000 cells ml−1) by species with respect to water temperature in Chesapeake Bay and Virginia tidal tributaries. Box plots show the 25–75th quartiles of water temperatures where the bloom concentrations were found. The vertical line within the box plot equals the median water temperature where the blooms occurred. The lower and upper error bars represent the minimum and maximum temperatures where bloom abundances were found. Data are compiled from harmful algal monitoring programs supported by the Virginia Department of Health, Virginia Department of Environmental Quality, and the Hampton Roads Sanitation District
To better understand the nutrient dynamics over varying timescales in a eutrophic environment during a succession of dinoflagellate blooms, we conducted a multi-year study during which we measured nutrient concentrations and uptake of inorganic and organic N and C compounds by dinoflagellate-dominated estuarine assemblages. We conducted these experiments at a site in the Lafayette River on a variety of timescales (seasonal, near-daily, and diurnal) over the course of blooms during 2002, when there were near-drought conditions, and during 2003, when the region received near-record rainfall. In subsequent years, we sampled daily (2005, 2006, and 2009) for periods of up to 52 days (Egerton et al. 2014; Morse et al. 2014) to observe dinoflagellate species succession during different seasons and with respect to nutrient availability.
Materials and Methods
We conducted a series of experiments at a single station in the Lafayette River, a sub-tributary of the James River and lower Chesapeake Bay (Fig. 2), during periods when there were high chlorophyll a (Chl a) concentrations (> 10 mg Chl L−1) due to monospecific (> 95% of the phytoplankton biomass was a single species) dinoflagellate blooms (2002 and 2003) and in non-bloom periods (2003). During 2002 and 2003, we focused on contrasting patterns in N and C uptake among bloom species and during bloom versus non-bloom conditions. In subsequent years, we sampled daily during periods when algal blooms normally develop. In 2005 and 2006, we sampled daily from the same stationary floating dock, and in 2009, we sampled from a bridge located near the site where the floating dock had been fixed.
Sampling Strategy
In 2002, we sampled blooms of P. minimum in April and May, an assemblage dominated by A. sanguinea and Skeletonema costatum during May, one dominated by A. sanguinea in August, and a bloom of C. polykrikoides in September during late morning. During 2003, we sampled blooms of H. triquetra in February and April, P. minimum in April and May, and A. sanguinea in June during late morning. In 2003, we also sampled during the late morning in July–September when communities were dominated by dinoflagellates but no one species dominated. We increased our sampling frequency in subsequent years collecting water samples daily at the same point in the tidal cycle to observe species succession in the Lafayette River during Spring, Summer, and Fall (Egerton et al. 2014; Morse et al. 2014).
To relate blooms of different dinoflagellate species to hydrographic properties and nutrient concentrations and uptake, we measured temperature, salinity, Chl a, and particulate N (PN) and C (PC) along with the associated natural abundance of 15N and 13C, DIN, which included ammonium [NH4+] and nitrate plus nitrite [NO3− + NO2−], urea, dissolved free amino acids (DFAA), total dissolved N (TDN), DON, DOC, and soluble reactive phosphate (PO43−). During 2002, 2005, and 2006, we measured uptake of NH4+, NO3−, urea N, and DFAA-N using stable isotopes as tracers. In 2005 and 2006, we also measured NO2− concentrations and its uptake. In 2002, we compared rates of N uptake with rates of bicarbonate (HCO3−) uptake, while in 2003, we determined the relative uptake of N and C from inorganic (NH4+, NO3−, and HCO3−) and organic compounds (urea, DFAA, and glucose) over the course of blooms and over diel cycles during blooms of P. minimum and A. sanguinea. For diel studies, populations were sampled at intervals over six 24-h periods, three for each species. N and C uptake was compared during bloom and non-bloom periods when Chl a concentrations were similar but the population was not dominated by any single algal species.
Sample Collection and Handling
Surface water samples were collected from a dock in acid-cleaned carboys and transported to Old Dominion University. Within 15 min of sample collection, nutrient samples were filtered through 0.2-μm Supor filters and the filtrate was placed into acid-cleaned sample bottles and frozen until analysis. Samples for Chl a were filtered onto Whatman glass fiber filters (GF/F) (pore size ~ 0.7 μm) and frozen until analysis. Samples were analyzed within 2 weeks of collection using the method of Welschmeyer (1994). For diel studies in 2003 and daily sampling in 2005 and 2006, whole water samples were preserved with acid Lugol’s solution for microscopic phytoplankton cell enumeration.
Whole water was placed into 50-mL acid-cleaned polycarbonate culture bottles to measure N and C uptake. Uptake experiments were initiated by adding tracer concentrations (0.03–0.3 μmol N L−1; usually > 1 and < 10% of the ambient nutrient pool) of highly enriched (96–99%) 15NH4+, 15NO3−, 15NO2−, 15N-13C-urea, 15N-13C-alanine, and 13C-glucose. The atom percent enrichment of the nutrient pool varied among dates in 2002 and 2003 ranging from 2.0 to 11.9% for glucose, 1.0 to 21.6% for NH4+, 1.0 to 41.3% for NO3−, 1.1 to 11.2% for urea N, 1.8 to 9.2% for urea C, 2.8 to 84.2% for amino acid N, and 3.1 to 57.4% for amino acid C. During 2005 and 2006, isotope additions for uptake experiments were always 0.1 μmol L−1. Because nutrient concentrations were at or near the limit of analytical detection on some sampling dates in 2005 (Morse et al. 2014), the atom percent enrichment exceeded 10% for all uptake experiments on August 16, for urea experiments on September 26, and for urea and NH4+ uptake experiments on September 28. In contrast, high DIN concentrations on September 20 resulted in atom % enrichments of < 2% on that date (but greater than 1%). Similarly, high NO2− concentrations in September resulted in atom % enrichments of < 2% on all of those sampling dates (but greater than 1%). In May 2006, ambient nutrient concentrations were near or below the limit of analytical detection for most of the dissolved N compounds measured (Egerton et al. 2014); the atom percent enrichments for NH4+ uptake experiments were 10.5–16.9%, while those for NO3− and urea uptake experiments were 66% and 70.1–81.7%, respectively. During both 2005 and 2006, amino acid N concentrations were estimated to be approximately 0.23 μM N (the average concentration that was measured in 2003; range 0.14–0.40 μM N) and therefore atom percent enrichments for all experiments were calculated to be 30%. For H13CO3−, additions were 200 μmol L−1 yielding enrichments ranging from 13 to 30%. Atom percent enrichments were calculated based on ambient salinity and temperature and the assumption that DIC was saturated.
After isotope additions were made, bottles were incubated at 20−22 °C under fluorescent lighting supplied at 30 to 35 μmol quanta m−2 s−1. After 15 to 30 min (1–2 h for H13CO3−), experiments were terminated by gentle filtration through precombusted (450 °C for 2 h) GF/C filters, and filters were frozen until analysis. GF/C filters have a nominal pore size of 1.2 μm and therefore should retain fewer bacteria than GF/F filters (nominal pore size of 0.7 μm) while retaining most phytoplankton.
We also conducted experiments to compare uptake rates measured using H13CO3− versus the more traditional 14C method using H14CO3− in natural water samples collected from the Lafayette River and the lower Chesapeake Bay near the mouth of the James River. For these experiments, we set up triplicate light and dark bottles for each substrate. We conducted for H13CO3− incubations as described above, but instead of 1–2-h incubations, we conducted sets of triplicate light and dark incubations that were terminated after 2, 4, 6, 12, and 24 h. For H14CO3− experiments, we added 2.5 μCi of aqueous Na2H14CO3 solution (PerkinElmer No. NEC-086H005MC, 5 mCi, pH < 9.5) to triplicate light and dark bottle incubations and the entire contents of the incubation bottles were filtered onto polycarbonate filters, rinsed with filtered estuarine water, and then placed into scintillation vials and fumed for 24 h with 0.5 mL of 0.5 N HCl. Ten millimeters of Fisher ScintiVerse BD scintillation cocktail was added and samples were counted on a Beckman scintillation counter. Rates were calculated as based on protocols established for the Joint Global Ocean Flux Study (JGOFS; IOC-SCOR 1994).
Analyses
Dissolved nutrients (NO3−, NO2−, urea, and PO43+) were measured colorimetrically using an Astoria Pacific nutrient analyzer according to the manufacturer’s specifications or manually (Parsons et al. 1984). NH4+ concentrations were measured colorimetrically using the phenol hypochlorite method (Solarzano 1969). DFAA were measured using a Shimadzu high performance liquid chromatography (HPLC) system (Cowie and Hedges 1992). TDN was analyzed after persulfate oxidation (Solorzano and Sharp 1980). DON was calculated as the difference between TDN and DIN. DOC was measured using the methods of Burdige et al. (1999). Glucose concentrations were estimated to be 2% of the DOC pool; glucose has been estimated to be 2–6% of the total DOC pool in surface waters (Benner 2002).
Samples destined for isotopic analyses were dried and pelletized into tin disks. They were then analyzed on a Europa isotope ratio mass spectrometer (IRMS) equipped with an automated N and C analyzer (ANCA). The IRMS was tuned daily to achieve maximum sensitivity and stability. Blank runs were performed to ensure that there was no system contamination. Calibration curves were run (6–8 standards) using a sucrose/ammonium sulfate solution. 15N and 13C atom percent values of our sucrose/ammonium sulfate standards were established using the National Institute of Standards and Technology certified standards. Standards were in the mass ranges of 1.17 to 100 μg N and 9.38 to 800 μg C. If the R2 was < 99% over the mass range for the standard curve, samples were not run. Samples with masses outside of these ranges were discarded. If the standard deviation of the isotope ratio of reference standards was > 0.0005 atom % for either N or C, samples were not run. We used this upper limit to establish detection limits of ≤ 0.0015 atom % for our samples. Sample batches were prepared with reference and reference check samples inserted after every eight samples to ensure that both the reference mass and the reference atom percent remained stable throughout the runs and to drift correct results.
Absolute uptake rates were calculated using a mixing model (Montoya et al. 1996) using the following equations and as described in Mulholland and Lee (2009):
While we attempted to enrich substrate pools by about 10%, this generally was not the case because nutrient concentrations varied by an order of magnitude even on a daily basis. This is not uncommon in estuarine environments (Egerton et al. 2014; Morse et al. 2014). When nutrient concentrations were at or near the limit of analytical detection, the atom percent enrichment of the substrate pool was greater than 10% on many occasions, uptake could have been stimulated, and uptake rates potentially overestimated. When nutrient concentrations were high, atom percent enrichments were low. To be conservative, when the atom percent enrichment was < 2.0%, uptake rates were not calculated. This is a level sufficient for accurate calculation of uptake rates at nutrient concentrations > 0.3 μM (Fig. 2 in Mulholland et al. 2009a).
Detection limits for the mass spectrometer were calculated for each sample run by determining the standard deviation of the atom percent values from the associated standard run and multiplying this by 3. The detection limit was always ≤ 0.0015 atom %. If the atom percent excess of a sample (atom percent enrichment of the PN pool after incubation minus the atom percent of the PN pool prior to enrichment) was less than the detection limit for a given sample run, uptake was assumed to be below the limit of analytical detection. We consider this a conservative method for estimating uptake rates. Detection limits for absolute uptake rates scale with particulate N and C concentration and so are sample-specific. Due to the large variability in the PN and PC concentrations over the course of this study, we did not calculate sample-specific detection limits for absolute uptake rates. The atom percent (or natural abundance of 15N and 13C) of the ambient particulate pool varied among sample dates and so was measured on each date.
The ambient N and C contribution from the DFAA pool was calculated based on the composition of the DFAA pool as described by Mulholland et al. (2002). We assumed the lability of all amino acids were equal. In the few cases where there were no DFAA measurements, the DFAA N and C concentrations were estimated based on the average DFAA concentration and relative N and C concentrations measured in samples collected under comparable conditions.
N- and C-specific growth rates were calculated from PN and PC turnover times estimated from total N and C uptake and the logistic growth equation.
Results
During Spring and early Summer 2002, the southeastern USA, including Virginia and much of the Chesapeake Bay watershed, experienced near-drought conditions. In contrast, there were record rainfalls during that time period in 2003. Consequently, salinities in the Lafayette River were higher in April and May 2002, ranging from 20 to 21, than during the same months in 2003, when salinities ranged from 8 to 18 (Table 1).
Despite these contrasting physical and meteorological conditions, monospecific algal blooms occurred during both years. In 2002, monospecific populations of P. minimum dominated the phytoplankton assemblage during two sampling dates in the spring, A. sanguinea was dominant on five sampling dates during spring and summer, and C. polykrikoides dominated the phytoplankton assemblage on the sampling date in late summer. Chlorophyll a concentrations ranged from 10.3 to 27.0 μg L−1, while PN and PC ranged from 13.5 to 95.8 μmol N L−1 and 92.9 to 602.7 μmol C L−1, respectively, during 2002 (Table 1). When P. minimum was the dominant dinoflagellate, PC:Chl a ratios were 3.6 to 4.7; however, when A. sanguinea dominated, this ratio ranged from 10.1 to 22.3. The PC:Chl a was highest (42.8) during a C. polykrikoides bloom in September.
During 2003, monospecific blooms were sampled more intensively and we also sampled during non-bloom periods when the phytoplankton assemblage was more diverse. Consequently, there was a much wider range in Chl a concentrations (7.2 and 159.4 μg L−1) and PN and PC concentrations (14.3 to 339.8 μmol N L−1 and 75.7 to 1509.3 μmol C L−1) at our sampling site (Table 1). Monospecific blooms of H. triquetra were sampled in winter and spring, P. minimum in spring, and A. sanguinea in early summer. Monospecific dinoflagellate blooms were not observed later in the summer during 2003. The PC:Chl a ratio ranged from 3.1 to 31.7 with the highest ratios observed during the latter part of a P. minimum bloom on May 6 and 8 (24.7 and 31.7, respectively). The lowest PC:Chl a ratios (3.1 to 10) were observed during periods when there were mixed phytoplankton assemblages during summer months.
DIN concentrations varied by an order of magnitude during both 2002 and 2003 ranging from 1.09 to 11.24 μmol L−1 during 2002 and 0.49 to 8.91 μmol L−1 during 2003 (Table 2). No seasonal patterns in DIN concentrations could be discerned in either year. Urea and DFAA concentrations varied within a relatively small range compared to DIN over both years, 0.24 to 1.98 μmol L−1 and 0.16 to 1.09 μmol L−1, respectively, and comprised a relatively small fraction of the DON pool. DON concentrations varied by a factor of 3, ranging from 17.2 to 63.7 μmol L−1 and were higher during late summer in both years. DOC concentrations ranged from 84.1 to 987.4 μmol L−1 and were also highest during the late summer months during both years. Phosphate concentrations ranged from 0.02 to 3.09 μmol L−1 and were generally higher during the summer months for both years.
N and C Uptake
During 2002, absolute N uptake rates ranged from 0.15 to 4.62 μmol N L−1 h−1 (Fig. 3), while Chl a-normalized N uptake rates ranged from 0.007 to 0.215 μmol N (μg Chl a) −1 h−1 (Table 2). The lowest N uptake rates were observed during the P. minimum bloom in the early spring; higher rates were observed during blooms of A. sanguinea and C. polykrikoides in the late spring and summer. All of the N compounds tested (NH4+, NO3−, urea, and DFAA) were taken up; however, the dominant N source varied both within and between blooms. For A. sanguinea, the relative contributions of the different N compounds to total measured N uptake varied on each sampling date.
During the more extensive sampling in 2003, absolute uptake rates ranged from 0.29 to 9.60 μmol N L−1 h−1 (Fig. 4) and Chl a-specific N uptake rates ranged from 0.007 to 0.748 μmol N (μg Chl a)−1 h−1 (Table 2). The highest N uptake rates were measured during the H. triquetra bloom in February and at the end of the P. minimum bloom in May when NO3− and urea uptake were especially high. Nitrogen uptake rates ranged from 0.29 to 5.41 μmol N L−1 h−1 on dates when no single dinoflagellate-dominated the phytoplankton assemblage and these rates were within the range observed during blooms in both 2002 and 2003. As for 2002, all of the N compounds tested were taken up during blooms in 2003; however, uptake rates of NH4+ were usually lower than those for NO3− and urea. During non-bloom periods in 2003, NH4+ contributed substantially to N uptake. In general, N uptake from DFAA contributed less to the total observed N uptake in 2003 but uptake rates were comparable to those measured in 2002.
We estimated turnover times for PN based on total measured N uptake and these averaged 0.78 day (± 0.31 day, excluding April 19) in 2002 and 0.89 day (± 0.77 day; range of 0.2–2.9 day excluding February 19) in 2003. The average N-based growth rates were 1.05 day−1 (± 0.54 day−1; range of 0.55–2.15 day−1) in 2002 and 1.45 day−1 (± 1.16 day−1; range of 0.24–3.93 day−1) in 2003.
Total measured C uptake rates ranged from 0.23–4.22 μmol C L−1 h−1 (Fig. 3) and 0.013 to 1.09 μmol C (μg Chl a)−1 h−1 (Table 2) during 2002. Bicarbonate uptake was measured on five of these dates and assimilation numbers ranged from 0.002 to 1.53 gC (g Chl a)−1 h−1. The highest bicarbonate uptake rates and assimilation numbers were during the A. sanguinea bloom in May (Fig. 3). During 2003, photosynthetic C uptake was measured on each sampling date and assimilation numbers ranged from 0.01 to 3.86 gC (g Chl a)−1 h−1 (Table 2). When uptake of all C compounds measured were included, Chl a-specific C uptake increased from 0.1 to 22.5 gC (g Chl a)−1 h−1. However, the total measured C uptake was less than what would be expected based on N uptake and Redfield stoichiometry (average Redfield stoichiometry of 6.6:1 C/N) (Table 2).
Carbon uptake rates were highly variable during both bloom and non-bloom periods. The highest C uptake rates were measured during blooms of P. minimum and A. sanguinea when rates of organic C uptake were high (Fig. 4). However, uptake of C associated with organic compounds (glucose, urea, and DFAA) also exceeded photosynthetic bicarbonate uptake during non-bloom periods, with the exception of two dates (Fig. 4).
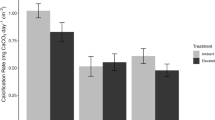
Because bicarbonate uptake was such a small fraction of the total C uptake, we examined whether primary productivity was underestimated using 13C versus 14C-labeled bicarbonate as a tracer. We found that C uptake rates measured using 13C were not significantly different than those made using the more traditional 14C method (Fig. 5).
The turnover time of PC due to photosynthesis ranged from 5.8 to 625 days during 2002 and 2003 (excluding an outlier of 1570 in 2002 when photosynthetic C uptake was very low) and on average was 110 days (± 145 days). This translated into an average growth rate of 0.031 day−1 (± 0.040 day−1). When uptake of C from organic compounds was included, the average PC turnover time was 7.5 days (± 8.01 days; range of 0.2–33 days, excluding two outliers of 72 and 222 days). The average C-based growth rate estimate was 0.35 day−1 (± 0.59 day−1) for the particulate C pool, closer to the N-based growth rate estimates above.
Diel Studies
Chl a, PC, and PN concentrations and the abundance of bloom organisms varied by up to 3 orders of magnitude over diurnal cycles during P. minimum and A. sanguinea blooms in 2003 (Table 3), likely due to the advection or vertical migration of cells. On April 30–May 1, P. minimum cell densities ranged from 682 to 238,000 cells mL−1 and Chl a concentrations ranged from 5.27 to 187 μg L−1 over a 24-h period. The highest cell abundance was observed during the middle of the day at about 2 pm. During the P. minimum bloom, the PC:Chl a ratio ranged from 10.7 to 33.2 (μmol C (μg Chl a)−1) and there was no consistent pattern with respect to the diel light cycle (Table 3). For the A. sanguinea blooms, cell abundance, Chl a, PN, and PC concentrations were all higher (by up to a factor of 10) during the day than during the night, but the PC:Chl a ratio varied over a much smaller range (6.7 to 13.5 μmol C (μg Chl a)−1).
Nutrient concentrations also varied on a diel basis. For NH4+, higher concentrations were observed in the late afternoon or at night when algal biomass was lower (with one exception on May 6, 2003) (Tables 3 and 4). While there was diel variability in NO3− concentrations during A. sanguinea blooms in June and July 2003, urea, DON, and PO43− concentrations did not vary much over the diurnal cycles during this study.
Diel variability in biomass had a profound effect on N uptake rates in surface waters; uptake rates generally, but not always, lower during the night than during the day. During the 2003 P. minimum bloom, all of the N compounds tested were taken up with urea and NO3− accounting for the bulk of N uptake most of the time (Fig. 6). NO3− uptake was limited to the light period during one of the diurnal studies (May 6), but on the other two, dark NO3− uptake was substantial. Urea, DFAA, and NH4+ were taken up during the day and at night (Fig. 6). Uptake of urea and DFAA N was higher than DIN uptake at night during two of the three diurnal studies. The relatively low NH4+ uptake rates during the P. minimum bloom were surprising given that NH4+ was present at comparable or higher concentrations than NO3− or urea on two of the diel study dates (Table 4).
Photosynthetic C uptake was detectable but only a small component of the total measured C uptake and limited to daylight hours (Fig. 6). Assimilation numbers were low ranging from 0.03 to 1.88 gC (g Chl a)−1 h−1. Absolute, Chl a- and cell-normalized dissolved organic C uptake far exceeded bicarbonate uptake during the day and night but were in good agreement with each other. The majority of the organic C uptake measured was from DFAA and urea but glucose uptake was also observed.
Similar to P. minimum, all of the N compounds tested were taken up by A. sanguinea over the three diurnal cycles examined and N uptake rates were lower during the night when cell abundance, Chl a and PN were also lower (Fig. 7) were less pronounced. Like P. minimum, A. sanguinea took up NO3− during both the day and night. On June 17, DIN uptake increased at night and exceeded uptake of DON. The reverse was true on June 19 when DIN uptake decreased at night and uptake of N from DFAA and urea increased. In July, DIN and DON uptake rates were comparable during the day and night. High concentrations of NH4+ and NO3− (Table 4) may have stimulated their uptake by A. sanguinea during blooms in June and July (Fig. 7). While Chl a-normalized N uptake rates by A. sanguinea were lower than those observed for P. minimum, likely because P. minimum has less chlorophyll per cell than A. sanguinea; volumetric and cell-normalized rates were comparable (Figs. 6 and 7).
Photosynthetic C uptake by A. sanguinea was limited to daylight hours but uptake of C from DFAA, urea, and glucose represented the bulk of the total C uptake over the diurnal light cycle (Fig. 7). Only on June 19 was DIC the dominant form of C taken up. However, assimilation numbers were very low ranging from 0.02 to 0.17 gC (g Chl a)−1 h−1. High glucose uptake rates were observed during the dark period on June 17. However, on June 19 and July 22, overall C uptake rates were lower and urea and DFAA-C uptake contributed the most to the total measured C uptake.
When comparing the relative N and C uptake from urea (C/N ratio of 0.5) and DFAA (as alanine; C/N ratio of 3.0) during P. minimum and A. sanguinea blooms in 2003 (Table 5), we found that the C/N uptake ratio from urea and DFAA were not stoichiometrically balanced relative to their abundance in each compound and that C was often preferentially taken up from these compounds.
Dinoflagellate Succession
Subsequent daily sampling during three seasons highlighted clear seasonal successional patterns of dinoflagellate species over the spring (2006), early summer (2009), and late summer/early fall (2005) (Fig. 8). Consistent with 2003, a Heterocapsa species (H. rotundata) bloomed in spring 2006 followed by a bloom of Gymnodinium instriatum in May. Similar to 2002 and 2003, A. sanguinea populations emerged in the summer in both 2005 and 2009. In early summer 2009, we observed a transition in the dinoflagellate community composition with A. sanguinea dominant in early June, S. trochoidea in late June and early July, and C. polykrikoides later in July. A mixed group of dinoflagellates dominated the phytoplankton community during late Summer and Fall in 2005. As for 2002 and 2003, there was no single form of N taken up during blooms in 2005 and 2006 (Fig. 8 insets). However, NH4+ satisfied about half the N demand during a G. instriatum bloom in Spring and NO2− satisfied over half of the N demand during blooms of Gymnodinium sp. in late Summer. Photosynthetic C uptake fulfilled virtually all of the C demand during the G. instriatum bloom in Spring and about half the C demand during the Gymnodinium sp. bloom in late Summer and early Fall (Fig. 8).
Dinoflagellate succession in the Lafayette River during spring 2006 (upper panel; redrawn from Egerton et al. 2014), early summer 2009 (middle panel; redrawn from Morse et al. 2013), and late summer/early fall 2005 (lower panel; redrawn from Morse et al. 2014). Panel insets are nitrogen and carbon uptake rates measured made on dates when dinoflagellate abundances were high. The asterisk indicates that NO2− uptake data were not available
Discussion
Timescales of Variability
During each year, we sampled in the Lafayette River, dinoflagellates dominated the phytoplankton assemblage, and monospecific blooms developed. While temperature appeared to be a primary control on the dinoflagellate assemblage and the species most likely to bloom at a particular time (Fig. 1), during this multi-year study, we found that there were interannual differences in dinoflagellate species succession and the timing and occurrence of blooms. These interannual differences were likely driven by climatological factors affecting hydrographic properties (e.g., water temperature and freshwater inputs) and nutrient inputs, as has been observed in other systems (Boneillo and Mulholland 2014). When observations were made daily in Spring (2006), early Summer (2009), and late Summer/early Fall (2005) (Fig. 8), species successions were observed in more detail and could be better related to shorter term variability in local meteorology, physical forcing, nutrient concentrations, and their interactions (Egerton et al. 2014; Morse et al. 2013, 2014).
In addition to interannual and daily to weekly variability driven by climatological factors, there were large fluctuations in surface nutrient and chlorophyll a concentrations and cell abundance over diurnal timescales during blooms. During the P. minimum bloom that was sampled six times over a 24-h period, Chl a and cell abundances varied by 2 to 3 orders of magnitude between early afternoon and midnight (Table 3). This could have been due to vertical migration of cells, fine-scale patchiness, tidal advection, or a combination of these. P. minimum is known to vertically migrate at rapid rates (Olsson and Granéli 1991). Regardless of the reason for this variability, these large fluctuations on such short timescales have important implications for water quality management and monitoring programs aimed at assessing water quality. The federal Environmental Protection Agency in the USA requires states to develop nutrient criteria for surface waters. In Virginia, the Department of Environmental Quality has established river segment- and season-specific numeric Chl a standards (currently 10–12 μg L−1 in the meso- and polyhaline segments of the lower James River estuary) that will be used to set total maximum daily loads to meet federal water quality requirements. To assess long-term status and trends of Chl a in the estuary, the Virginia Chesapeake Bay Monitoring Program samples fixed stations, monthly, in fair weather, during the day. However, in the present study we observed interannual, seasonal, daily, and even diurnal variability in algal biomass that was not captured in the monthly monitoring records compiled over a decade (Fig. 9). Together, these results suggest that in dynamic systems such as the Lafayette River estuary, where ephemeral blooms exert short-term variability far in excess of that reported from routine monthly monitoring, better assessment tools are necessary to assess water quality impairments.
Nutrient Concentrations
Concentrations of DIN and DON were also highly variable over weekly, daily, and diurnal timescales, as has been observed in this system previously (Table 4; Egerton et al. 2014; Morse et al. 2014). This is likely because nutrient loading is highly sporadic and closely related to tidal cycles and freshwater inputs from rainfall, storms, and coastal flooding (Gilbert et al. 2005; Filippino et al. 2017). As for algal biomass, short-term variability in nutrient concentrations may be greater than that observed in low frequency (e.g., monthly) monitoring data. While highly variable, DOC concentrations peaked in late summer (Table 2), similar to what was observed in a mid-Atlantic coastal bay with a long residence time and prone to brown tide blooms (Simjouw et al. 2004; Mulholland et al. 2009a, b, Boneillo and Mulholland 2014). Residence times of 1 to 4 months have been estimated for the Lafayette River, depending interannual variability in freshwater inputs (White 1972), and this long residence time may facilitate the accumulation of organic matter in this watershed, particularly in 2002 when rainfall was low in the region. Accumulation of organic matter in estuaries can be a source of nutrients fueling future water quality impairments even after nutrient loads from the surrounding landscape are reduced and may result in a lag time between load reduction and improvements in water quality.
Nitrogen Uptake
As has been shown in previous interannual comparisons (Boneillo and Mulholland 2014; Bronk et al. 2014), there was no single nitrogen compound that could be consistently associated with bloom activity each year (Figs. 3, 4, and 8). During all years, DIN contributed substantially to N demand during both bloom and non-bloom periods, similar to what has been shown in other estuarine and coastal marine systems (Heil et al. 2007; Mulholland and Lomas 2008; Mulholland et al. 2009a, b; Boneillo and Mulholland 2014; Bronk et al. 2014; Moschonas et al. 2017). While not measured in 2002 or 2003, during late Summer and Fall of 2005, NO2− was also a quantitatively large source of N to Gymnodinium sp. and other dinoflagellate-dominated phytoplankton communities (Fig. 8), as was observed during a C. polykrikoides bloom in 2007 (Mulholland et al. 2009b). Nitrite concentrations of up to 10 μM have been measured in the Lafayette estuarine system during late summer (Mulholland et al. 2009b, Morse et al. 2014) and were 5.4 and 6.7 μM on the two dates in September 2005, when uptake rates were measured (data not shown). The accumulation of NO2− is likely due to incomplete nitrification during late summer when there is mixing of NH4+-rich, low oxygen bottom waters with more oxygenated surface waters (McCarthy et al. 1984).
Despite high DIN concentrations, organic nitrogen was also an important source of nitrogen throughout this study. Urea and DFAA contributed significantly to the total measured N uptake during blooms (Figs. 3, 4, 6, 7, and 8), consistent with other studies (Glibert et al. 2001; Mulholland et al. 2002, 2009a, b; Heil et al. 2007; Anderson et al. 2008; Gobler et al. 2012; Boneillo and Mulholland 2014; Bronk et al. 2014; Moschonas et al. 2017). We also observed that high rates of DON uptake for these compounds were not limited to periods when there were blooms (Fig. 4). During the summer of 2003, when there were no monospecific blooms observed, but dinoflagellates still dominated the phytoplankton community, uptake of DON compounds usually exceeded that of DIN. Similar observations were made in another eutrophic system during summer in the absence of algal blooms (Boneillo and Mulholland 2014) and in coastal systems where the composition of the dissolved N pool has been related to phytoplankton community composition (Heil et al. 2007; Moschonas et al. 2017).
The dominant form of N taken up also varied over the course of individual blooms. For example, during two A. sanguinea blooms in 2002, the dominant source of N taken up on each of the five sampling dates was different; urea, NH4+, DFAA, and NO3−, were the dominant N compounds taken up on different days (Fig. 3). Similar variability in N uptake rates were observed during a C. polykrikoides bloom in 2007 (Mulholland et al. 2009b). In contrast, N uptake during the G. instriatum bloom in 2006 was always dominated by NH4+ on the 4 days it was measured. Most phytoplankton have the capacity to take up a diverse suite of N compounds, and the rate of N uptake by phytoplankton is thought to be regulated by the relative growth phase, physiological status of cells, temperature, and nutrient concentrations (Mulholland and Lomas 2008). However, changes in the dominant form of N taken up over the short time scales (e.g., days and diurnal cycles) have not been demonstrated frequently. This flexibility in nitrogen uptake may contribute to the success of bloom-forming phytoplankton in dynamic coastal environments, where the inputs of nitrogen are sporadic and comprised of diverse compounds, physical conditions are dynamic, and biological production and recycling exerts strong impacts on the biogeochemical cycling of nutrients. The physiological status of bloom populations varies over the course of blooms as they initiate, propagate, and decay, and nutrient concentrations are drawn down (Bronk et al. 2014; Killberg-Thoreson et al. 2014; Egerton et al. 2014; Morse et al. 2014)
In addition to short-term variability (e.g., daily) in N uptake during blooms, we observed interannual variability in the dominant form of N taken up during blooms of the same species. For example, in 2002, NH4+ contributed about 50% of the total N uptake in natural populations dominated by P. minimum (Fig. 3) consistent with the observations made during a bloom of P. minimum in the Choptank River (Fan et al. 2003). However, in 2003, urea and NO3− were the dominant N sources taken up during the P. minimum bloom in the Lafayette River (Fig. 4). This may have been because of interannual differences in nutrient availability. Interannual variability in the dominant N compound taken up was also observed in a multi-year study of A. anophagefferens blooms in a nearby coastal bay (Boneillo and Mulholland 2014) suggesting that the form of N present may be less important than the total quantity of bioavailable N (Davidson et al. 2012).
N uptake also varied over diurnal timescales during blooms of P. minimum and A. sanguinea during 2003. Most measurements of N uptake during blooms have been made during the day when uptake is thought to be energetically most favorable. We measured high rates of N uptake even during the night (Figs. 6 and 7) when cell abundance and Chl a biomass were lower (Table 3). Dark uptake of inorganic N (in particular, nitrate) is thought to be limited by the supply of energy and reductant from photosynthesis and dark uptake of inorganic N has been linked with nutrient limitation (MacIsaac 1978; Paasche et al. 1984; Kudela and Cochlan 2000; Fan and Glibert 2005). Results presented here suggest that night-time uptake is an important but overlooked means of meeting cellular N demands and this may contribute to the success of dinoflagellates in nutrient-rich environments. In a culture study, P. minimum took up N at night in deeper water and this was linked to their rapid vertical migration (Olsson and Granéli 1991). Because all of the incubations from the present study were done using surface water, we could not account for variations in cellular nutrient acquisition and metabolism as a result of vertical migration.
Based on observed N uptake rates, PN turnover times were rapid (0.78 ± 0.31 days in 2002 and 0.89 ± 0.77 days in 2003) suggesting that there were ample sources of N in this system to fuel rapid algal growth. The rapid uptake of N and turnover of biomass make it impossible to assess nutrient availability, preferences, and uptake from in situ dissolved nutrient concentrations. While allochthonous N inputs contribute substantially to N inputs in the Lafayette River, in situ N recycling of biomass and regeneration of sedimentary organic matter may also contribute substantially to N availability in this shallow eutrophic system. N regeneration rates were not measured as part of this study, but uptake and regeneration of NH4+ are tightly coupled in most aquatic systems (Bronk and Steinberg 2008; Bronk et al. 2014). If urea and DFAA, along with NH4+, were rapidly regenerated in our short-term incubation experiments, the uptake rates reported here would be underestimates (Lipschultz 2008).
Carbon Uptake and Osmotrophy
Phytoplankton are generally thought to be sources of DOC in the environment fueling bacterial growth (Carlson and Hansell 2015). However, in this study, we determined that H. triquetra, P. minimum, and A. sanguinea all took up C from dissolved organic compounds (urea, DFAA, and glucose), and DOC uptake exceeded bicarbonate uptake on most dates measured (Fig. 4). While urea and DFAA are generally thought of as N sources for phytoplankton growth (Mulholland and Lomas 2008), C uptake rates from these compounds were also high on many occasions. Urea and DFAA carbon uptake has been observed during blooms of the pelagophyte, A. anophagefferens (Mulholland et al. 2009a; Boneillo and Mulholland 2014) and during a bloom of C. polykrikoides in the lower James River estuary (Mulholland et al. 2009b). However, these compounds were used primarily as an N source in the Gulf of Mexico associated with blooms of Karenia brevis (Bronk et al. 2014) and in A. anophagefferens blooms in Quantuck Bay, NY (Lomas 2004; Mulholland et al. 2009a). The ratio of C/N taken up from urea and DFAA in the present study suggests that these compounds were used primarily as a C source during parts of the day (Table 5). Although we expected that organic C uptake would be higher at night when cells are not photosynthetically active, this was not always the case (Figs. 6 and 7). While we did not measure the bacterial contribution to the observed C uptake in this study, based on our calculations, phytoplankton accounted for > 99% of the C biomass in this system during blooms.
Assimilation numbers calculated from photosynthetic bicarbonate uptake ranged from 0.002 to 3.86 gC (g Chl a)−1 h−1 (Table 2) and were usually much lower than the range previously observed in phytoplankton cultures (Glover 1980) and in the Chesapeake Bay (Harding et al. 1985). While the mean assimilation number was similar to those reported during a bloom in another Chesapeake Bay tributary (0.62 gC (g Chl a)−1 h−1; Gallegos 1992) and those predicted for large dinoflagellates (1.29 to 1.50 gC (g Chl a)−1 h−1; Gallegos 1992), we generally observed extremely low assimilation numbers during both bloom and non-bloom periods. Assimilation numbers are widely used in models and remote sensing to convert phytoplankton biomass to productivity. However, assimilation numbers are highly variable as they are indicative of phytoplankton physiology and nutrient and light limitation (Parsons 2002; Milligan et al. 2015). Low assimilation numbers have been found in other systems where mixotrophs are abundant (Laybourn-Parry and Perriss 1997). Because heterotrophy and autotrophy require different biochemical machinery, it is thought that mixotrophs reduce their photosynthetic machinery when grazing (Sanders et al. 1990); however, others suggest that photosynthetic machinery might continue to provide energy during mixotrophic growth (Wilken et al. 2014). The exceedingly low assimilation numbers we observed here (Table 2) suggest that Chl a may have been retained by cells taking up organic C and has important implications for modeling productivity based on Chl a biomass in systems dominated by phytoplankton mixotrophs (Flynn and Mitra 2009; Flynn et al. 2012).
Particulate carbon turnover times estimated from bicarbonate uptake alone were much longer than those observed for nitrogen averaging 110 ± 145 days, resulting in a stoichiometric imbalance in the C/N turnover (Table 2). When we include the contribution of organic C uptake measured in this study, turnover times of the PC pool decreased to 7.5 ± 8.0 days, reducing this imbalance (Table 2) and closing the gap between N and C-based growth rate estimates (1.34 and 0.35 day−1, respectively). To examine whether this gap could be reconciled by integrating C and N uptake over diurnal timescales when light-dependent and light-independent uptake might vary, we conducted diurnal studies. During the P. minimum bloom in 2003, when we made rate measurements at six time points over a 24-h period, we applied each rate over 4 h of the diurnal cycle to calculate the total daily N and C uptake as 124.3 μmol N L−1 h−1 and 585.5 μmol C L−1 h−1, a C/N uptake ratio of 4.7, and closer to Redfield stoichiometry. This suggests unbalanced growth, missing C sources, or that growth is balanced over longer timescales. In the present study, C uptake was measured for only a limited suite of organic C compounds that together make up a very small fraction of the mostly uncharacterized DOC pool (Repeta 2014). If other DOC compounds are also taken up by cells, and we view this as likely, our estimated contributions of DOC to total measured C uptake are underestimates.
Advantages of Mixotrophy
Little is known about the role of DOC uptake in augmenting autotrophic metabolisms (Lewitus 2006; Burkholder et al. 2008), even though high DOC concentrations are often reported during and after blooms of algal mixotrophs (Gobler et al. 2004; Mulholland et al. 2009a; Boneillo and Mulholland 2014), uptake of organic N is thought to be common in the environment (Mulholland and Lomas 2008) and has been linked to algal blooms (Anderson et al. 2002; Heisler et al. 2008). DOM additions have been shown to stimulate algal growth; however, this growth stimulation is generally attributed to the DON (Doblin et al. 1999; Fagerberg et al. 2009; Loureiro et al. 2009; Filippino et al. 2011; Cawley et al. 2013).
In addition to osmotrophy, it is well-known that grazing by dinoflagellate mixotrophs can contribute to their nutrition (Jeong et al. 2005a, 2015; Burkholder et al. 2008). In a study of Alexandrium catenella, Collos et al. (2013) found that only 47% of the C demand was met by bicarbonate uptake and the remainder was made up through grazing. While we did not examine mixotrophic grazing in this study, many of the dinoflagellates that dominated the algal community in this study are known to graze (Li et al. 2000; Jeong et al. 2004, 2005a, b, 2015). Early studies suggested that algal heterotrophy was a response to low ambient nutrient concentrations (Stoecker et al. 1997, Fan et al. 2003, Stoecker and Gustafson 2003); however, more recently, algal mixotrophy has been found to be common in eutrophic environments (Adolf et al. 2008, Burkholder et al. 2008). The expression of trophic modes in dinoflagellates appears to be species-specific, thus allowing different species to exhibit diverse physiological responses to the nutritional and physical environments and the abundance of prey and thereby occupy a wide variety of nutritional niches (Stoecker 1999). For example, P. minimum grazed cryptophyte prey when inorganic nutrients were limited but not in response to low light suggesting that P. minimum feeds to obtain limiting nutrients rather than supplement their C nutrition (Stoecker et al. 1997). While mixotrophic grazing was not examined in the present study, osmotrophic uptake of dissolved organic compounds substantially subsidized both N and C acquisition during bloom and non-bloom periods and during the day and night and should be considered in mixotrophic models.
Our results suggest DOC uptake by dinoflagellates during the light and dark may contribute substantially to their C demand in eutrophic estuaries. This may be important because light availability can become limiting during blooms due to self-shading, shading by other particles, or when cells are advected or migrate out of the photic zone. When light is limited or at night, DOC uptake can supplement photosynthetic carbon fixation and support additional inorganic and organic N acquisition. The flexibility of dinoflagellates with regards to their N and C nutrition along with vertical migration (Olsson and Granéli 1991; Katano et al. 2011) may contribute to their success in the eutrophic estuaries where nutrient concentrations are dynamic and high algal density can result in light limitation. While osmotrophy and phagotrophy may augment the nutrition of primarily phototrophic dinoflagellates (e.g., P. minimum), offering them a competitive advantage over strict photoautotrophs, photosynthesis may augment the C nutrition of primarily heterotrophic dinoflagellates (e.g., A. sanguinea) offering them a competitive advantage over strictly heterotrophic protists in environments or at times when prey densities are low (Havskum and Hansen 1997; Stoecker et al. 1997; Stoecker 1999). Mixotrophy also allows organisms to supplement DIC uptake and acquire C and N over the entire diurnal light cycle in surface waters or in deeper water allowing them nutritional options over the entire day. These strategies may contribute to the ability of mixotrophic organisms to outcompete co-occurring phytoplankton and form blooms.
Conclusions
Our results suggest that dinoflagellates are metabolically versatile and this may contribute to their competitive success in highly variable, nutrient-rich estuarine environments. Bloom-forming dinoflagellates in the lower Chesapeake Bay have flexible N and C metabolisms that vary over short time scales and allow them to exploit the highly variable nutrient and hydrographic environment that results from tidal and freshwater forcing. The nutritional flexibility exhibited by dinoflagellates may contribute to the success of mixotrophic species in dynamic, eutrophic environments where there are abundant sources of organic and inorganic nutrients and timescales of variability are short; however, it may make them difficult to model if osmotrophy contributes to low and variable assimilation numbers. As found previously, there appears to be no single form of N that fuels blooms of dinoflagellates and the relative contribution of inorganic and organic N and C sources to their nutrition varies seasonally, over the course of individual blooms and over diurnal cycles. While in the traditional microbial loop, DOC produced by photosynthetic organisms is channeled to bacteria and then through the food web (Graneli et al. 1999), mixotrophic phytoplankton utilizing DOM may provide an alternative pathway for C flow in systems dominated by mixotrophic microbes. Further, because mixotrophic behaviors affect carbon flow through ecosystems (Flynn et al. 2012; Mitra et al. 2014), this may contribute to the observation that most estuaries are thought to be heterotrophic (Gattuso et al. 1998; Middelburg and Nieuwenhuize 2000) despite high algal biomass. Based on the metabolic theory that temperature affects heterotrophic processes more strongly than autotrophic processes, Wilken et al. (2013) found that the mixotroph Ochromonas sp. became more heterotrophic with rising temperatures. If this is true for other mixotrophic organisms, it will have important implications for food webs and carbon flow in estuarine systems dominated by mixotrophic microbes.
References
Adolf, J.E., T. Bachvaroff, and A.R. Place. 2008. Can cryptophyte abundance trigger toxic Karlodinium veneficum blooms in eutrophic estuaries? Harmful Algae 8 (1): 119–128.
Anderson, D.M., P.M. Glibert, and J.M. Burkholder. 2002. Harmful algal blooms and eutrophication: Nutrient sources, composition, and consequences. Estuaries 25 (4): 704–726.
Anderson, D.M., J.M. Burkholder, W.P. Cochlan, P.M. Glibert, C.J. Gobler, C.A. Heil, R.M. Kudela, M.L. Parsons, J.E.J. Rensel, and D.W. Townsend. 2008. Harmful algal blooms and eutrophication: Examining linkages from selected coastal regions of the United States. Harmful Algae 8 (1): 39–53.
Benner, R. 2002. Chemical composition and reactivity. In Biogeochemistry of marine dissolved organic matter, ed. D.A. Hansell and C.A. Carlson, 59–90. London: Academic Press.
Berg, G.M., J. Shrager, G. Glockner, K.R. Arrigo, and A.R. Grossman. 2008. Understanding nitrogen limitation in Aureococcus anophagefferens (Pelagophyceae) through cDNA and qRT-PCR analysis. Journal of Phycology 44 (5): 1235–1249.
Berman, T., and D.A. Bronk. 2003. Dissolved organic nitrogen: A dynamic participant in aquatic ecosystems. Aquatic Microbial Ecology 31: 279–305.
Boneillo, G.E., and M.R. Mulholland. 2014. Interannual differences in nutrient dynamics during brown tide (Aureococcus anophagefferens) blooms in a coastal embayment. Estuaries and Coasts 37 (Suppl 1): 147–163.
Bronk, D.A., L. Killberg-Thoreson, R.E. Sipler, M.R. Mulholland, Q.N. Roberts, P.W. Bernhardt, M. Garrett, J.M. O’Neil, and C.A. Heil. 2014. Nitrogen uptake and regeneration (ammonium regeneration, nitrification and photoproduction) in waters of the West Florida Shelf prone to blooms of Karenia brevis. Harmful Algae 38: 50–62.
Bronk, D.A., and D.K. Steinberg. 2008. Nitrogen Regeneration. In Nitrogen in the marine environment, ed. D.G. Capone, D.A. Bronk, M.R. Mulholland, and E.J. Carpenter, 385–467. New York: Academic Press.
Burdige, D.J., W.M. Berelson, J. McManus, K. Coale, and K. Johnson. 1999. Fluxes of dissolved organic carbon from California continental margin sediments. Geochimica Cosmochimica Acta 63 (10): 1507–1515.
Burkholder, J.M., P.M. Glibert, and H.M. Skelton. 2008. Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Harmful Algae 8 (1): 77–93.
Carlson, C.A., and D.A. Hansell. 2015. DOM sources, sinks, reactivity and budgets. In Biogeochemistry of marine dissolved organic matter, ed. D.A. Hansell and C.A. Carlson, 65–126. Amsterdam: Elsevier Publishers B.V.
Cawley, K.M., V. Koerfer, and D.M. McKnight. 2013. The role of dissolved organic matter (DOM) quality in the growth enhancement of Alexandrium fundyense (Dinophyceae) in laboratory culture. Journal of Phycology 49 (3): 546–554.
Charette, M.A., and K.O. Buessler. 2004. Submarine groundwater discharge of nutrients and copper to an urban subestuary of Chesapeake Bay (Elizabeth River). Limnology and Oceanography 49 (2): 376–385.
Collos, Y., C. Jauzein, M. Laabir, and A. Vaquei. 2013. Discrepancies between net particulate carbon production and 13C-labelled bicarbonate uptake by Alexandrium catenella (Dinophyceae): Grazing controls the balance between autotrophic and non-autotrophic carbon acquisition. Journal of Phycology 49 (3): 441–446.
Cowie, G.L., and J.I. Hedges. 1992. Improved amino acid quantification in environmental samples: Charge-matched recovery standards and reduced analysis time. Marine Chemistry 37 (3-4): 223–238.
Davidson, K., R.J. Gowen, P. Tett, E. Bresnan, P.J. Harrison, A. McKinney, S. Milligan, D.K. Mills, J. Silke, and A.-M. Crooks. 2012. Harmful algal blooms: How strong is the evidence that nutrient ratios and forms influence their occurrence? Estuarine, Coastal and Shelf Science 115: 399–413.
Doblin, M.A., S.I. Blackburn, and G.M. Hallegraeff. 1999. Growth and biomass stimulation of the toxic dinoflagellate Gymnodinium catenatum (Graham) by dissolved organic substances. Journal of Experimental Marine Ecology 236 (1): 33–47.
Dzurica, S., C. Lee, E.M. Cosper, and E.J. Carpenter. 1989. Role of environmental variables, specifically organic compounds and micronutrients, in the growth of the crysophyte Aureococcus anophagefferens, the “brown tide” microalga. In Novel phytoplankton blooms: Causes and impacts of recurrent brown tides and other unusual blooms, lecture notes on coastal and estuarine studies, ed. E.M. Cosper, V.M. Bricelj, and E.J. Carpenter, 229–252. Berlin: Springer-Verlag.
Egerton, T.A., R.E. Morse, H.G. Marshall, and M.R. Mulholland. 2014. The effects of short-term variability in water quality on phytoplankton abundance, diversity, and community composition in a tidal estuary. Microorganisms 2 (4): 33–57.
Fagerberg, T., P. Carlsson, and M. Lundgren. 2009. A large molecular size fraction of riverine high molecular weight dissolved organic matter (HMW DOM) stimulates growth of the harmful dinoflagellate Alexandrium minutum. Harmful Algae 8 (6): 823–831.
Fan, C., P.M. Glibert, and J.M. Burkholder. 2003. Characterization of the affinity for nitrogen, uptake kinetics, and environmental relationships for Prorocentrum minimum in natural blooms and laboratory cultures. Harmful Algae 2 (4): 283–299.
Fan, C., and P.M. Glibert. 2005. Effects of light on nitrogen and carbon uptake during a Prorocentrum minimum bloom. Harmful Algae 4 (3): 629–641.
Filippino, K.C., T.A. Egerton, W.S. Hunley, and M.R. Mulholland. 2017. The effects of two successive storms, Hurricane Irene and Tropical Storm Lee, on water quality in the tidal James River. Estuaries and Coasts 40 (1): 80–94.
Filippino, K.C., M.R. Mulholland, P.W. Bernhardt, N.G. Love, E. Canuel, M. Sanderson, and D.A. Bronk. 2011. Bioavailability of effluent-derived organic nitrogen to resident microbial communities along a natural estuarine salinity gradient. Estuaries and Coasts 34 (2): 269–280.
Flynn, K.J., and A. Mitra. 2009. Building the “perfect beast”: Modelling mixotrophic plankton. Journal of Plankton Research 31 (9): 965–992.
Flynn, K.J., D.K. Stoecker, A. Mitra, J.A. Raven, P.M. Glibert, P.J. Hansen, E. Graneli, and J.M. Burkholder. 2012. Misuse of the phytoplankton-zooplankton dichotomy: The need to assign organisms as mixotrophs within plankton functional types. Journal of Plankton Research 35: 3–11.
Gallegos, C.L. 1992. Phytoplankton photosynthesis, productivity, and species composition in a eutrophic estuary: Comparison of bloom and non-bloom assemblages. Marine Ecology Progress Series 81: 257–267.
Gattuso, J.-P., M. Frankignoulle, and R. Wollast. 1998. Carbon and carbonate metabolism in coastal aquatic ecosystems. Annual Review of Ecological Systems 29 (1): 405–434.
Glibert, P.M., R. Magnien, M.W. Lomas, J. Alexander, C. Fan, E. Haramoto, M. Trice, and T.M. Kana. 2001. Harmful algal blooms in the Chesapeake and coastal bays of Maryland, USA: Comparison of 1997, 1998, and 1999 events. Estuaries 24 (6): 875–883.
Glibert, P.M., S. Seitzinger, C.A. Heil, J.M. Burkholder, M.W. Parrow, L.A. Codispoti, and V. Kelly. 2005. The role of eutrophication in the global proliferation of harmful algal blooms. Oceanography 18 (2): 198–209.
Glover, H.E. 1980. Assimilation numbers in cultures of marine phytoplankton. Journal of Plankton Research 2 (1): 69–79.
Gobler, C.J., D.L. Berry, S.T. Dyhrman, S.W. Wilhelm, A. Salamov, A.V. Lobanov, Y. Zhang, J.L. Collier, L.L. Wurch, A.B. Kustka, B.D. Dill, M. Shah, N.C. VerBerkmoes, A. Kuo, A. Terry, J. Pangilinan, E. Lindquist, S. Lucas, I. Paulsen, T.K. Hattenrath, S.C. Talmage, E.A. Walker, F. Koch, A.M. Burson, M.A. Marcoval, Y.-Z. Tang, G.R. LeCleir, K.J. Coyne, G.M. Berg, E.M. Bertrand, M.A. Saito, V. Gladyshev, and I.V. Grigoriev. 2011. Niche of harmful alga Aureococcus anophagefferens revealed through ecogenomics. Proceedings of the National Academy of Sciences 108 (11): 4352–4357.
Gobler, C.J., S. Deonarine, J. Leigh-Bell, M.D. Gastrich, O.R. Anderson, and S.W. Wilhelm. 2004. Ecology of phytoplankton communities dominated by Aureococcus anophagefferens: The role of viruses, nutrients, and microzooplankton grazing. Harmful Algae 3 (4): 471–483.
Gobler, C.J., A. Burson, F. Koch, Y. Tang, and M.R. Mulholland. 2012. The role of nitrogenous nutrients in the occurrence of harmful algal blooms caused by Cochlodinium polykrikoides in New York estuaries (USA). Harmful Algae 17: 64–74.
Graneli, E., P. Carlsson, and C. Legrand. 1999. The role of C, N, and P in dissolved and particulate organic matter as a nutrient source for phytoplankton growth, including toxic species. Aquatic Ecology 33 (1): 17–27.
Hansen, P.J. 2011. The role of photosynthesis and food uptake for the growth of marine mixotrophic dinoflagellates. Journal of Eukaryotic Microbiology 58 (3): 203–214.
Harding, L.W., Jr., B.W. Meeson, and T.R. Fisher Jr. 1985. Photosynthesis patterns in Chesapeake Bay phytoplankton: Short- and long-term responses of P-I curve parameters to light. Marine Ecology Progress Series 26: 99–111.
Havskum, H., and A.S. Hansen. 1997. Importance of pigmented and colourless nano-sized protists as grazers on nanoplankton in a phosphate-depleted Norwegian fjord and in enclosures. Aquatic Microbial Ecology 12: 139–151.
Heil, C.A., M. Revilla, P.M. Glibert, and S. Murasko. 2007. Nutrient quality drives differential phytoplankton community composition on the Southwest Florida shelf. Limnology and Oceanography 52 (3): 1067–1078.
Heisler, J., P.M. Glibert, J.M. Burkholder, D.M. Anderson, W. Cochlan, W.C. Dennison, Q. Dortch, C.J. Gobler, C.A. Heil, E. Humphries, A. Lewitus, R. Magnien, H.G. Marshall, K. Sellner, D.A. Stockwell, D.K. Stoecker, and M. Suddleson. 2008. Eutrophication and harmful algal blooms: A scientific consensus. Harmful Algae 8 (1): 3–13.
IOC-SCOR. 1994. Protocols for the joint global ocean fluxes study (JGOFS) core measurements IOC manual and guides 29. Paris: UNESCO.
Jeong, H.J., Y.D. Yoo, J.S. Kim, T.H. Kim, J.H. Kim, N.S. Kang, and W. Yih. 2004. Mixotrophy in the phototrophic harmful alga Cochlodinium polykrikoides (Dinophycean): Prey species, the effects of prey concentration, and grazing impact. Journal of Eukaryotic Microbiology 51 (5): 563–569.
Jeong, H.J., J.Y. Park, J.H. Nho, M.O. Park, J.H. Ha, K.A. Seong, C. Jeng, C.N. Seong, K.Y. Lee, and W.H. Yih. 2005a. Feeding by red-tide dinoflagellates on the cyanobacterium Synechococcus. Aquatic Microbial Ecology 41: 131–143.
Jeong, H.J., Y.D. Yoo, J.Y. Park, J.Y. Song, S.T. Kim, S.H. Lee, K.Y. Kim, and W.H. Yih. 2005b. Feeding by phototrophic red-tide dinoflagellates: Five species newly revealed and six species previously known to be mixotrophic. Aquatic Microbial Ecology 40: 133–150.
Jeong, H.J., A.S. Lim, P.J.S. Franks, K.H. Lee, J.H. Kim, N.S. Kang, M.J. Lee, S.H. Jang, S.Y. Lee, E.Y. Yoon, J.Y. Park, Y.D. Yoo, K.A. Seong, J.E. Kwon, and T.Y. Jang. 2015. A hierarchy of conceptual models of red-tide generation: Nutrition, behavior, and biological interactions. Harmful Algae 47: 97–115.
Katano, T., M. Yoshida, S. Yamaguchi, T. Hamada, K. Yoshino, and Y. Hayami. 2011. Diel vertical migration and cell division of bloom-forming dinoflagellate Akashiwo sanguinea in the Ariake Sea, Japan. Plankton and Benthos Research 6 (2): 92–100.
Killberg-Thoreson, L., M.R. Mulholland, C. A. Heil, M. P. Sanderson, J. M. O’Neil, and D. A. Bronk. 2014. Nitrogen uptake kinetics in field populations and cultured strains of Karenia brevis. Harmful Algae 38:73–85.
Kim, S., A Kuo, and J. Kwon. 2002. A model study of flushing characteristics of the Elizabeth River, Virginia. Estuarine and coastal modeling (2001). In Estuarine and Coastal Modeling, ed., M. L. Spaulding, 643–653. American Society of Civil Engineers.
Kudela, R.M., and W.P. Cochlan. 2000. Nitrogen and carbon uptake kinetics and the influence of irradiance for a red tide bloom off southern California. Aquatic Microbial Ecology 21: 31–47.
Laybourn-Parry, J., and S.J. Perriss. 1997. A mixotrophic ciliate as a major contributor to plankton photosynthesis in Australian lakes. Limnology and Oceanography 42 (6): 1463–1467.
Lewitus, A.J. 2006. Osmotrophy in marine microalgae. In Algal cultures, analogues and blooms, ed. D.V. Subba Rao, 343–383. New Hampshire: Science Publishers, Inc.
Li, A., D.K. Stoecker, and D.W. Coats. 2000. Spatial and temporal aspects of Gyrodinium galatheanum in Chesapeake Bay: Distribution and mixotrophy. Journal of Plankton Research 22 (11): 2105–2124.
Li, J., P.M. Glibert, and Y. Gao. 2015. Temporal and spatial changes in Chesapeake Bay water quality and relationships to Prorocentrum minimum, Karlodinium veneficum, and CyanoHAB events, 1991–2008. Harmful Algae 42: 1–14.
Lipschultz, F. 2008. Isotope tracer methods for studies of the marine nitrogen cycle. In Nitrogen in the marine environment, ed. D.G. Capone, D.A. Bronk, M.R. Mulholland, and E.J. Carpenter, 1345–1384. New York: Academic Press.
Lomas, M. W. 2004. Does urea-carbon contribute significantly to Aureococcus anophagefferens carbon nutrition? In Proceedings of the 10th International HAB Meeting, 402–404. Cape Town, South Africa.
Loureiro, S., E. Garces, Y. Collos, D. Vaque, and J. Camp. 2009. Effect of marine autotrophic dissolved organic matter (DOM) on Alexandrium catenella in semi-continuous cultures. Journal of Plankton Research 31 (11): 1363–1372.
MacIsaac, J. 1978. Diel cycles of inorganic nitrogen uptake in a natural phytoplankton population dominated by Gonyaulax polyedra. Limnology and Oceanography 23 (1): 1–9.
Marshall, H.G. 1968. Plankton in James River Estuary, Virginia III. Phytoplankton in the Lafayette and Elizabeth Rivers (Western and Eastern Branches). Castanea 33: 255–258.
Marshall, H. G. 1995. Succession of dinoflagellate blooms in the Chesapeake Bay USA. In Harmful Marine Algal Blooms, eds., P. Lassus, G. Arzul, E. Erard-Le Denn, P. Gentien, and M. Marcillou-Le Baut, 615–620. Lavoisier.
Marshall, H.G., M.F. Lane, and K.K. Nesius. 2003. Long-term phytoplankton trends and related water quality trends in the lower Chesapeake Bay, Virginia, U.S.A. Environmental Monitoring and Assessment 81 (1/3): 349–360.
Marshall, H.G., L. Burchardt, and R. Lacouture. 2005. A review of phytoplankton composition within Chesapeake Bay and its tidal estuaries. Journal of Plankton Research 27 (11): 1083–1102.
Marshall, H.G., M.F. Lane, K.K. Nesius, and L. Burchardt. 2009. Assessment and significance of phytoplankton species composition within Chesapeake Bay and Virginia tributaries through a long-term monitoring program. Environmental Monitoring and Assessment 150 (1-4): 143–155.
McCarthy, J.J., W. Kaplan, and J.L. Nevins. 1984. Chesapeake Bay nutrient and plankton dynamics. 2. Sources and sinks of nitrite. Limnology and Oceanography 29 (1): 84–98.
Middelburg, J.J., and J. Nieuwenhuize. 2000. Nitrogen uptake by heterotrophic bacteria and phytoplankton in the nitrate-rich Thames estuary. Marine Ecology Progress Series 203: 13–21.
Milligan, A. J., K. H. Halsey, and M. J. Behrenfeld. 2015. Advancing interpretations of 14C-uptake measurements in the context of phytoplankton physiology and ecology. Journal of Plankton Research 37: 692–698.
Mitra, A., K.J. Flynn, J.M. Burkholder, T. Berge, A. Calbet, J.A. Raven, E. Graneli, P.M. Glibert, P.J. Hansen, D.K. Stoecker, F. Thingstad, U. Tillmann, S. Vage, S. Wilken, and M.V. Zubkov. 2014. The role of mixotrophic protists in the biological carbon pump. Biogeosciences 11 (4): 995–1005.
Montoya, J.P., M. Voss, P. Kaehler, and D.G. Capone. 1996. A simple, high precision tracer assay for dinitrogen fixation. Applied Environmental Microbiology 62 (3): 986–993.
Morse, R.E., J. Shen, J.L. Blanco-Garcia, W.S. Hunley, S. Fentress, M. Wiggins, and M.R. Mulholland. 2011. Environmental and physical controls on the formation and transport of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in lower Chesapeake Bay and its tributaries. Estuaries and Coasts 34 (5): 1006–1025.
Morse, R.E., M.R. Mulholland, W.S. Hunley, S. Fentress, M. Wiggins, and J.L. Blanco-Garcia. 2013. Controls on the initiation and development of blooms of the dinoflagellate Cochlodinium polykrikoides Margalef in lower Chesapeake Bay and its tributaries. Harmful Algae 28: 71–82.
Morse, R.E., M.R. Mulholland, T.E. Egerton, and H.G. Marshall. 2014. Daily variability in phytoplankton abundance and nutrient concentrations in a tidally dominated eutrophic estuary. Marine Ecology Progress Series 503: 59–74.
Moschonas, G., R.J. Gowen, R.F. Paterson, E. Mitchell, B.M. Stewart, S. McNeill, P.M. Glibert, and K. Davidson. 2017. Nitrogen dynamics and phytoplankton community structure: The role of organic nutrients. Biogeochemistry 134 (1-2): 125–145.
Mulholland, M.R., C.J. Gobler, and C. Lee. 2002. Peptide hydrolysis, amino acid oxidation and N uptake in communities seasonally dominated by Aureococcus anophagefferens. Limnology and Oceanography 47 (4): 1094–1108.
Mulholland, M.R., and C. Lee. 2009. Peptide hydrolysis and dipeptide uptake in cultures and natural communities dominated by phytoplankton mixotrophs. Limnology and Oceanography 54 (3): 856–868.
Mulholland, M.R., and M.W. Lomas. 2008. N uptake and assimilation. In Nitrogen in the marine environment, ed. D.G. Capone, D.A. Bronk, M.R. Mulholland, and E.J. Carpenter, 303–384. New York: Academic Press.
Mulholland, M.R., G.E. Boneillo, P.W. Bernhardt, and E.C. Minor. 2009a. Comparison of nutrient and microbial dynamics over a seasonal cycle in a mid-Atlantic coastal lagoon prone to Aureococcus anophagefferens (brown tide) blooms. Estuaries and Coasts 32 (6): 1176–1194.
Mulholland, M.R., R.E. Morse, G.E. Boneillo, P.W. Bernhardt, K.C. Filippino, L.A. Procise, J.L. Blanco-Garcia, H.G. Marshall, T.A. Egerton, W.S. Hunley, K.A. Moore, D.L. Berry, and C.J. Gobler. 2009b. Understanding causes and impacts of the dinoflagellate, Cochlodinium polykrikoides, blooms in the Chesapeake Bay. Estuaries and Coasts 32 (4): 734–747.
Olsson, P., and E. Granéli. 1991. Observations on diurnal vertical migration and phased cell division for three coexisting marine dinoflagellates. Journal of Plankton Research 13 (6): 1313–1324.
Paasche, E., I. Bryceson, and K. Tangen. 1984. Interspecific variation in dark nitrogen uptake by dinoflagellates. Journal of Phycology 20 (3): 394–401.
Parsons, T.R., Y. Maita, and C. Lalli. 1984. A manual of chemical and biological methods for seawater analysis. Oxford: Pergamon Press.
Parsons, T.R. 2002. On the use of 14C measurements of primary production. Limnology and Oceanography Bulletin 11: 75.
Repeta, D.J. 2014. Chemical characterization and cycling of dissolved organic matter. In Biogeochemistry of marine dissolved organic matter, ed. D.A. Hansell and C.A. Carlson, 22–64. Amsterdam: Elsevier Publishers B.V.
Sanders, R.W., K.G. Porter, and D.A. Caron. 1990. Relationship between phototrophy and phagotrophy in the mixotrophic chrysophyte Poterioochromonas malhamensis. Microbial Ecology 19 (1): 97–109.
Simjouw, J.P., M.R. Mulholland, and E.C. Minor. 2004. Molecular-level characteristics of dissolved organic matter in Chincoteague Bay prior to and during an Aureococcus anophagefferens bloom. Estuaries 27 (6): 986–998.
Solarzano, L. 1969. Determination of ammonia in natural waters by the phenol hypochlorite method. Limnology and Oceanography 14: 16–23.
Solorzano, L., and J.H. Sharp. 1980. Determination of total dissolved phosphorus and particulate phosphorus in natural waters. Limnology and Oceanography 25: 752–754.
Stoecker, D.K. 1999. Mixotrophy among dinoflagellates. Journal of Eukaryotic Microbiology 46 (4): 397–401.
Stoecker, D.K., A. Li, D.W. Coats, D.E. Gustafson, and M.K. Nannen. 1997. Mixotrophy in the dinoflagellate Prorocentrum minimum. Marine Ecology Progress Series 152: 1–12.
Stoecker, D.K., and D.E. Gustafson. 2003. Cell-surface proteolytic activity of photosynthetic dinoflagellates. Aquatic Microbial Ecology 30: 175–183.
Tittel, J., V. Bissinger, B. Zippel, U. Gaedke, E. Bell, A. Lorke, and N. Kamjunke. 2003. Mixotrophs combine resource use to outcompete specialists: Implications for aquatic food webs. Proceedings of the National Academy of Sciences 100 (22): 12776–12781.
Welschmeyer, N.A. 1994. Fluorometric analysis of chlorophyll a in the presence of chlorophyll b and pheopigments. Limnology and Oceanography 39 (8): 1985–1992.
White, E. G. 1972. A physical hydrographic study of the Lafayette River. M.S. Thesis, Norfolk, VA, USA: Old Dominion University.
Wilken, S., J. Huisman, S. Naus-Wiezer, and E. Van Donk. 2013. Mixotrophic organisms become more heterotrophic with rising temperature. Ecology Letters 16 (2): 225–233.
Wilken, S., J.M. Schuurmans, and H.C.P. Matthijs. 2014. Do mixotrophs grow as photoheterotrophs? Photophysiological acclimation of the chrysophyte Ochromonas Danica after feeding. New Phytologist 204 (4): 882–889.
Acknowledgements
This work was funded by a Virginia Environmental Endowment and National Science Foundation grants to Margaret R. Mulholland. We wish to thank Michelle Watson who contributed to sampling efforts during 2002 and 2003 and George Boneillo, Andrea Rocha, and Old Dominion University Water Quality Laboratory for their help in analyzing samples. We thank two anonymous reviewers for their constructive comments on an earlier version of this manuscript.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Paul A. Montagna
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Mulholland, M.R., Morse, R., Egerton, T. et al. Blooms of Dinoflagellate Mixotrophs in a Lower Chesapeake Bay Tributary: Carbon and Nitrogen Uptake over Diurnal, Seasonal, and Interannual Timescales. Estuaries and Coasts 41, 1744–1765 (2018). https://doi.org/10.1007/s12237-018-0388-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12237-018-0388-5