Abstract
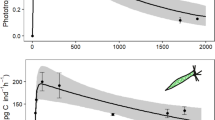
The time scales involved in the transition between phototrophic and phagotrophic modes of nutrition were examined in the mixotrophic chrysophytePoterioochromonas malhamensis. Phagotrophy began almost immediately when bacteria were added to phototrophically growing cultures of the alga, and chlorophylla concentration per cell in these cultures decreased over a 24-hour period. Chlorophyll concentrations per cell began to increase when bacteria were grazed to a density of approximately 106 ml−1, but after more than 24 hours they had not returned to the higher chlorophyll concentrations observed in the phototrophically grown cultures. Bacterivory was the dominant mode of nutrition in all cultures containing heat-killed bacteria. Photosynthesis did not contribute more than ≈7% of the total carbon budget of the alga when in the presence of abundant heat-killed bacteria. Bacterial density was the primary factor influencing the ability ofP. malhamensis to feed phagotrophically, while light intensity, pH, and the presence of dissolved organic matter had no effect on phagotrophy. We conclude thatP. malhamensis is capable of phagotrophy at all times. In contrast, phototrophy is inducible in the light during starvation and is a long-term survival strategy for this mixotrophic alga (i.e., it operates on time scales greater than a diel cycle).
Similar content being viewed by others
References
Aaronson S, Baker H (1959) A comparative biochemical study of two species ofOchromonas. J Protozool 6:282–284
Andersson A, Falk S, Samuelsson G, Hagström Å (1989) Nutritional characteristics of a mixotrophic nanoflagellate,Ochromonas sp. Microb Ecol 17:252–262
Bird DF, Kalff J (1986) Bacterial grazing by planktonic algae. Science 231:493–495
Bird DF, Kalff J (1987) Algal phagotrophy: Regulating factors and importance relative to photosynthesis inDinobryon (Chrysophyceae). Limnol Oceanogr 32:277–284
Bird DF, Kalff J (1989) Phagotrophic sustenance of a metalimnetic phytoplankton peak. Limnol Oceanogr 34:155–162
Caron DA, Porter KG, Sanders RW (1990) Carbon, nitrogen and phosphorus budgets for the mixotrophic phytoflagellatePoterioochromas malhamensis (Chrysophyceae) during bacterial ingestion. Limnol Oceanogr (in press)
Davis PG, Sieburth JMcN (1982) Differentiation of the phototrophic and heterotrophic nanoplankton populations in marine waters by epifluorescence microscopy. Ann Inst Oceanogr Paris 58[suppl]:249–261
Estep KW, Davis PG, Hargraves PE, Sieburth JM (1984) Chloroplast containing microflagellates in natural populations of North Atlantic nanoplankton, their identification and distribution; including a description of five new species ofChrysochromulina (Prymnesiophyceae). Protistologica 20:613–634
Estep KW, Davis PG, Keller M, Sieburth JM (1986) How important are oceanic algal nan-oflagellates in bacterivory? Limnol Oceanogr 31:646–650
Hutner SH, Provasoli L, Filfus J (1953) Nutrition of some phagotrophic fresh-water chrysomonads. Ann NY Acad Sci 56:852–862
Myers J, Graham J (1956) The role of photosynthesis in the physiology ofOchromonas. J Cell Comp Physiol 47:397–414
Parsons TR, Maita Y, Lalli CM (1984) A manual of chemical and biological methods for seawater analysis. Pergamon Press, New York
Porter KG (1988) Phagotrophic phytoflagellates in microbial food webs. Hydrobiologia 159:89–97
Porter KG, Feig YS (1980) The use of DAPI for identifying and counting aquatic microflora. Limnol Oceanogr 25:943–948
Sanders RW, Porter KG (1988) Phagotrophic phytoflagellates. In: Marshall K (ed) Advances in microbial ecology, vol. 10. Plenum Press, New York, pp 167–192
Sanders RW, Porter KG, Bennett SJ, DeBiase AE (1989) Seasonal patterns of bacterivory by flagellates, ciliates, rotifers, and cladocerans in a freshwater planktonic community. Limnol Oceanogr 34:673–687
Sherr BF, Sherr EB, Fallon RD (1987) Use of mono-dispersed, fluorescently labeled bacteria to estimate in situ protozoan bacterivory. Appl Environ Microbiol 53:958–965
Starr RC (1978) The culture colleciton of algae at the University of Texas at Austin. J Phycol 14[suppl]:47–100
Stein JR (1973) Handbook of phycological methods. Cambridge Press, Cambridge
Tranvik L, Porter KG, Sieburth JM (1989) Occurrence of bacterivory inCryptomonas, a common freshwater phytoplankter. Oecologia 78:473–476
Wetzel R, Likens GE (1979) Limnological analyses. WB Saunders Co., Philadelphia
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Sanders, R.W., Porter, K.G. & Caron, D.A. Relationship between phototrophy and phagotrophy in the mixotrophic chrysophytePoterioochromonas malhamensis . Microb Ecol 19, 97–109 (1990). https://doi.org/10.1007/BF02015056
Issue Date:
DOI: https://doi.org/10.1007/BF02015056