Abstract
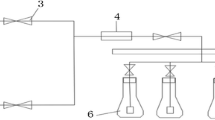
Carbon dioxide (CO2) transfer in the intensive cultivation of microalgae is a crucial process in photobioreactor performance. This study evaluated three operating conditions (bubble size, aeration rate, and CO2 concentration) to improve the growth performance of the microalga Tetradesmus obliquus in a laboratory–scale photobioreactor. Two types of air diffusers were used (glass pipette and a sintered glass diffuser), three aerations rates (0.125, 0.25 and 0.5 vvm), and four CO2–enriched air concentrations (0.04, 0.5, 1.0 and 2.0%) were investigated during the Tetradesmus obliquus cultivations. The results showed that the overall gas-liquid mass transfer coefficient (kLa) CO2 can be raised by increasing the aeration rate and using a sintered glass diffuser; however, CO2 capture efficiency was lower when the highest aeration rates were applied. When the glass diffuser was used at an aeration rate of 0.25 vvm, a kLa CO2 of 11.98 ± 0.6 1/h was provided, in comparison to 4.90 ± 0.19 1/h for the use of pipette at 0.5 vvm (maximum value reached). Similarly, the highest CO2 capture efficiency rate (67.94 ± 3.56%) was found applying an aeration rate of 0.25 vvm. At a CO2 concentration of 1 or 2% the T. obliquus biomass reached approximately 4.3 g/L, values significantly higher (p < 0.05) than the values reported for supplementation of 0.5% (~ 3.9 g/L) and 0.04% (~ 1.5 g/L). In summary, to avoid losses of CO2 to the atmosphere, an addition of 1% CO2 at an aeration rate of 0.25 vvm using a sintered glass diffuser were the optimal conditions to be applied in cylindrical laboratory–scale photobioreactor for T. obliquus growth.



Similar content being viewed by others
Data Availability
Data will be made available on request.
References
Prasad R, Gupta SK, Shabnam N, Oliveira CYB, Nema AK, Ansari FA, Bux F (2021) Role of microalgae in global CO2 sequestration: physiological mechanism, recent development, Challenges, and future prospective. Sustainability 13(23):13061. https://doi.org/10.3390/su132313061
Tai TC, Calosi P, Gurney-Smith HJ, Cheung WW (2021) Modelling ocean acidification effects with life stage-specific responses alters spatiotemporal patterns of catch and revenues of american lobster, Homarus americanus. Sci Rep 11(1):23330. https://doi.org/10.1038/s41598-021-02253-8
Oliveira CYB, Jacob A, Nader C, Oliveira CDL, Matos ÂP, Araújo ES, Shabnam N, Ashok B, Gálvez AO (2022) An overview on microalgae as renewable resources for meeting sustainable development goals. J Environ Manag 320:115897. https://doi.org/10.1016/j.jenvman.2022.115897
Cheng L, Zhang L, Chen H, Gao C (2006) Carbon dioxide removal from air by microalgae cultured in a membrane-photobioreactor. Sep Purif Technol 50:324–329. https://doi.org/10.1016/j.seppur.2005.12.006
Verma R, Srivastava A (2018) Carbon dioxide sequestration and its enhanced utilization by photoautotroph microalgae. Environ Develop 27:95–106. https://doi.org/10.1016/j.envdev.2018.07.004
Ojah A, Sabri LS, Aldahhan MH (2021) Local volumetric mass transfer coefficient estimation for Scenedesmus microalgae culture in a cylindrical airlift photobioreactor. J Chem Technol Biotechnol 96(3):764–774. https://doi.org/10.1002/jctb.6590
Tebbani S, Filali R, Lopes F, Dumur D, Pareau D (2014) CO2 biofixation by microalgae: modeling, estimation and control. John Wiley & Sons, Oxford
Langley N, Harrison S, van Hille R (2012) A critical evaluation of CO2 supplementation to algal systems by direct injection. Biochem Eng 68:70–75. https://doi.org/10.1016/j.bej.2012.07.013
de Godos I, Mendoza J, Acién F, Molina E, Banks C, Heaven S, Rogalla F (2014) Evaluation of carbon dioxide mass transfer in raceway reactors for microalgae culture using flue gases. Bioresour Technol 153:307–314. https://doi.org/10.1016/j.biortech.2013.11.087
Goldman JC, Dennett MR, Riley CB (1981) Inorganic carbon sources and biomass regulation in intensive microalgal cultures. Biotechnol Bioeng 23:995–1014. https://doi.org/10.1002/bit.260230508
Chia SR, Nomanbhay SBHM, Chew KW, Munawaroh HSH, Show PL (2022) Algae as potential feedstock for various bioenergy production. Chemosphere 287:131944. https://doi.org/10.1016/j.chemosphere.2021.131944
ElFar OA, Chang CK, Leong HY, Peter AP, Chew KW, Show PL (2021) Prospects of industry 5.0 in algae: customization of production and new advance technology for clean bioenergy generation. Energy Convers Manag X 10:100048. https://doi.org/10.1016/j.ecmx.2020.100048
Contreras A, Garcia F, Molina E, Merchuk JC (1998) Interaction between CO2-mass transfer, light availability, and hydrodynamic stress in the growth of Phaeodactylum tricornutum in a concentric tube airlift photobioreactor. Biotechnol Bioeng 60:317–325. https://doi.org/10.1002/(SICI)1097-0290(19981105)60:3<317::AID-BIT7>3.0.CO;2-K
Talbot P, Gortares MP, Lencki RW, de la Noüe J (1991) Absorption of CO2 in algal mass culture systems: a different characterization approach. Biotechnol Bioeng 37:834–842. https://doi.org/10.1002/bit.260370907
Talbot P, Lencki RW, de la Noüe J (1990) Carbon dioxide absorption characterization of a bioreactor for biomass production of Phormidium bohneri: comparative study of three types of diffuser. J Appl Phycol 2:341–350. https://doi.org/10.1007/BF02180924
Ying K, Al-Mashhadani MKH, Hanotu JO, Gilmour DJ, Zimmerman WB (2013) Enhanced mass transfer in microbubble driven airlift bioreactor for microalgal culture. Engineering 5:735–743. https://doi.org/10.4236/eng.2013.59088
Oliveira CYB, Viegas TL, da Silva MFO, Fracalossi DM, Lopes RG, Derner RB (2020) Effect of trace metals on growth performance and accumulation of lipids, proteins, and carbohydrates on the green microalga Scenedesmus obliquus. Aquac Int 28(4):1435–1444. https://doi.org/10.1007/s10499-020-00533-0
Lee JY, Hong ME, Chang WS, Sim SJ (2015) Enhanced carbon dioxide fixation of Haematococcus pluvialis using sequential operating system in tubular photobioreactors. Process Biochem 50:1091–1096. https://doi.org/10.1016/j.procbio.2015.03.021
Tang D, Han W, Li P, Miao X, Zhong J (2011) CO2 biofixation and fatty acid composition of Scenedesmus obliquus and Chlorella pyrenoidosa in response to different CO2 levels. Bioresour Technol 102:3071–3076. https://doi.org/10.1016/j.biortech.2010.10.047
Chisti Y, Jauregui-Haza UJ (2002) Oxygen transfer and mixing in mechanically agitated airlift bioreactors. Biochem Eng J 10(2):143–153. https://doi.org/10.1016/S1369-703X(01)00174-7
Fan LH, Zhang YT, Zhang L, Chen HL (2008) Evaluation of a membrane-sparged helical tubular photobioreactor for carbon dioxide biofixation by Chlorella vulgaris. J Membrane Sci 325:336–345. https://doi.org/10.1016/j.memsci.2008.07.044
Merchuk JC, Gluz M, Mukmenev I (2000) Comparison of photobioreactors for cultivation of the red microalga Porphyridium sp. J Chem Technol Biotechnol 75:1119–1126. https://doi.org/10.1002/1097-4660(200012)75:12<1119::aid-jctb329>3.0.co;2-g
Wang C, Lan CQ (2018) Effects of shear stress on microalgae–a review. Biotechnol Adv 36(4):986–1002. https://doi.org/10.1016/j.biotechadv.2018.03.001
Wetzel RG, Likens GE (2010) Limnological analyses. Springer, New York
Thielmann J, Tolbert NE, Goyal A, Senger H (1990) Two systems for concentrating CO2 and bicarbonate during photosynthesis by Scenedesmus. Plant Physiol 92:622–629. https://doi.org/10.1104/pp.92.3.622
Moroney JV (1999) How do algae concentrate CO2 to increase the efficiency of photosynthetic carbon fixation? Plant Physiol 119:9–16. https://doi.org/10.1104/pp.119.1.9
Yang Y, Gao K (2003) Effects of CO2 concentrations on the freshwater microalgae, Chlamydomonas reinhardtii, Chlorella pyrenoidosa and Scenedesmus obliquus (Chlorophyta). J Appl Phycol 15:379–389. https://doi.org/10.1023/a:1026021021774
Azov Y (1982) Effect of pH on inorganic carbon uptake in algal cultures. Appl Environ Microbiol 43:1300–1306
de Morais MG, Costa JAV (2007) Biofixation of carbon dioxide by Spirulina sp. and Scenedesmus obliquus cultivated in a three-stage serial tubular photobioreactor. J Biotechnol 129:439–445. https://doi.org/10.1016/j.jbiotec.2007.01.009
Yoo C, Jun SY, Lee JY, Ahn CY, Oh HM (2010) Selection of microalgae for lipid production under high levels carbon dioxide. Bioresour Technol 101:71–74. https://doi.org/10.1016/j.biortech.2009.03.030
Ho SH, Chen CY, Chang JS (2012) Effect of light intensity and nitrogen starvation on CO2 fixation and lipid/carbohydrate production of an indigenous microalgae Scenedesmus obliquus CNW-n. Bioresour Technol 113:244–252. https://doi.org/10.1016/j.biortech.2011.11.133
Kaewkannetra P, Enmak P, Chiu T (2012) The effect of CO2 and salinity on the cultivation of Scenedesmus obliquus for biodiesel production. Biotechnol Bioproc E 17:591–597. https://doi.org/10.1007/s12257-011-0533-5
Ho SH, Chen WM, Chang JS (2010) Scenedesmus obliquus CNW-n as a potential candidate for CO2 mitigation and biodiesel production. Bioresour Technol 101:8725–8730. https://doi.org/10.1016/j.biortech.2010.06.112
Satoh A, Kurano N, Miyachi S (2001) Inhibition of photosynthesis by intracellular carbonic anhydrase in microalgae under excess concentrations of CO2. Photosynth Res 68:215–224. https://doi.org/10.1023/a:1012980223847
Chiu SY, Kao CY, Chen CH, Kuan TC, Ong SC, Lin CS (2008) Reduction of CO2 by a high-density culture of Chlorella sp. in a semicontinuous photobioreactor. Bioresour Technol 99:3389–3396. https://doi.org/10.1016/j.biortech.2007.08.013
Singh S, Singh P (2014) Effect of CO2 concentration on algal growth: a review. Renew Sust Energ 38:172–179. https://doi.org/10.1016/j.rser.2014.05.043
Märkl H (1977) CO2 transport and photosynthetic productivity of a continuous culture of algae. Biotechnol Bioeng 19:1851–1862. https://doi.org/10.1002/bit.260191209
Zhang K, Kurano N, Miyachi S (2002) Optimized aeration by carbon dioxide gas for microalgal production and mass transfer characterization in a vertical flat-plate photobioreactor. Bioproc Biosyst Eng 25:97–101. https://doi.org/10.1007/s00449-002-0284-y
Chiu SY, Tsai MT, Kao CY, Ong SC, Lin CS (2009) The air-lift photobioreactors with flow patterning for high-density cultures of microalgae and carbon dioxide removal. Eng Life Sci 9:254–260. https://doi.org/10.1002/elsc.200800113
Martínez-Jerónimo F, Espinosa-Chávez F (1994) A laboratory-scale system for mass culture of freshwater microalgae in polyethylene bags. J Appl Phycol 6:423–425. https://doi.org/10.1007/bf02182159
Leong YK, Chew KW, Chen WH, Chang JS, Show PL (2021) Reuniting the biogeochemistry of algae for a low-carbon circular bioeconomy. Trends Plant Sci 26(7):729–740. https://doi.org/10.1016/j.tplants.2020.12.010
Oliveira CYB, Oliveira CDL, Prasad R, Ong HC, Araujo ES, Shabnam N, Gálvez AO (2021) A multidisciplinary review of Tetradesmus obliquus: a microalga suitable for large-scale biomass production and emerging environmental applications. Rev Aquac 13(3):1594–1618. https://doi.org/10.1111/raq.12536
Fu J, Huang Y, Xia A, Zhu X, Zhu X, Chang JS, Liao Q (2022) How the sulfur dioxide in the flue gas influence microalgal carbon dioxide fixation: from gas dissolution to cells growth. Renew Energy 198:114–122. https://doi.org/10.1016/j.renene.2022.08.057
Funding
This study received financial support from the Brazilian Ministry of Science, Technology, Innovation, and Communications–MCTIC/CGTS/SETEC provided by the Funding Authority for Studies and Projects (FINEP) (Agreement No. 01.10.0457.00) and the National Council for Scientific and Technological Development (CNPq) (Case No. 407513/2013-2).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
CRediT Authorship Contribution Statement
FRFP, RGL, HC, CN and RGC developed the experimental design and conducted the data acquisition and analysis. FRFP, HC, CN and CYBO wrote the manuscript, and all authors conducted the data interpretation and revised the manuscript.
Competing Interests
The authors declare that they have no conflict of interest.
Ethical Approval
This article does not contain any studies with animals performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
da Fonseca Pchara, F.R., Cella, H., Nader, C. et al. Investigating Carbon Dioxide Transfer for Intensive Cultures of the Microalga Tetradesmus obliquus. Bioenerg. Res. 17, 547–556 (2024). https://doi.org/10.1007/s12155-023-10622-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-023-10622-6