Abstract
Microalgal cultures are usually sparged with CO2-enriched air to preclude CO2 limitation during photoautotrophic growth. However, the CO2 vol% specifically required at operating conditions to meet the carbon requirement of algal cells in photobioreactor is never determined and 1–10% v/v CO2-enriched air is arbitrarily used. A scheme is proposed and experimentally validated for Chlorella vulgaris that allows computing CO2-saturated growth feasible at given CO2 vol% and volumetric O2 mass-transfer coefficient (k L a)O. CO2 sufficiency in an experiment can be theoretically established to adjust conditions for CO2-saturated growth. The methodology completely eliminates the requirement of CO2 electrode for online estimation of dissolved CO2 to determine critical CO2 concentration (Ccrit), specific CO2 uptake rate (SCUR), and volumetric CO2 mass-transfer coefficient (k L a)C required for the governing CO2 mass-transfer equation. Ccrit was estimated from specific O2 production rate (SOPR) measurements at different dissolved CO2 concentrations. SCUR was calculated from SOPR and photosynthetic quotient (PQ) determined from the balanced stoichiometric equation of growth. Effect of light attenuation and nutrient depletion on biomass estimate is also discussed. Furthermore, a simple design of photosynthetic activity measurement system was used, which minimizes light attenuation by hanging a low depth (ca. 10 mm) culture over the light source.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the developments in microalgal biotechnology towards the production of sustainable and clean bioenergy, it is becoming increasingly important to improve the kinetics and yield of microalgal cultures. Low cell density and productivity heavily constrain the utilization of algae to its full potential (Eriksen 2008; Ugwu et al. 2008). Understanding the microalgal culture is challenging, but vital for improving the process performance. Light, which drives the photochemical reactions to produce usable energy for carbon fixation in algal photosynthesis, has been researched utmost to maximize the rate of photosynthesis in photobioreactors, where the cells are often light-limited due to self shading (Olivieri et al. 2014). Mathematical models of photosynthesis–light relationship, description of light regime (periodic variations in light intensity experienced by individual cells as they traverse through light gradient inside culture), and prediction of photosynthetic response to light regime are the cornerstones of photobioreactor engineering (Brindley et al. 2011; Fernández et al. 1997; Molina-Grima et al. 1999; Merchuk et al. 2007; Pottier et al. 2005; Pruvost et al. 2008; Yun and Park 2003). Carbon dioxide—the carbon source—is another crucial factor affecting photoautotrophic growth. The effect of CO2 concentration on the rate of photosynthesis has been known since the pioneering experiments of the British plant physiologist F. F. Blackman in 1905. Like the depletion of oxygen in aerobic cultures, CO2 may get limiting with the increase in algal cell density under inappropriate operating conditions.
Although the CO2 is far more soluble in water than oxygen, the equilibrium concentration of CO2 in water at 25 °C and 1 atm is ca. 0.6 ppm and that of O2 is 15 times, since the volume percent of CO2 in air (0.04%) is negligible compared to O2 (21%). In addition, presence of salts in growth medium can reduce solubility of the gas. Microalgal cultures in PBRs, therefore, are usually sparged with CO2-enriched air (1–10% v/v CO2, generally 5%) to avoid carbon limitation. However, this practice has a serious shortcoming which has been overlooked. Sparging the culture with CO2-enriched air cannot ensure that the cells will never be CO2-limited throughout the course of cultivation. Analogous to critical O2 concentration for aerobically respiring cells, microalgal cells will not be CO2-limited as long as the dissolved CO2 concentration is maintained above the critical CO2 concentration (Ccrit). Below Ccrit, the specific CO2 uptake rate (SCUR) should decrease. It implies that to maintain non-limiting concentration of CO2 in microalgal cultures, Ccrit should be determined and dissolved CO2 should be maintained above Ccrit. Apparently, there are at least three technical problems associated with the determination of Ccrit and maintaining the dissolved CO2 above it. First, measuring SCUR at different dissolved CO2 concentrations to determine Ccrit and monitoring dissolved CO2 require online measurement of dissolved CO2 with CO2 electrode. Unlike the ease in measuring dissolved oxygen (DO) with common oxygen electrodes integrated in bioreactors, sterilizable CO2 electrodes are uncommon and expensive. Second, even if the CO2 electrode is available, the study could be perplexing, as the rate of photosynthesis, and hence the rate of carbon fixation, is not only dependent on dissolved CO2 but also largely on light intensity. Third, since the growth of microalgae is very slow, it is likely that the SCUR measurements could be very long in duration. However, the experiments for the measurement of SCUR must be short for the same reason which requires short experiments for measurement of specific oxygen uptake rate for aerobically respiring cells. These inherent difficulties in the measurement of Ccrit probably prevented algal researchers to determine Ccrit and to assure CO2 saturation by maintaining dissolved CO2 greater than Ccrit. Rather, sparging the microalgal culture with an optimized level of CO2 vol% or an arbitrary high CO2 level is a common practice. Microalgal cells will be CO2-saturated throughout the course of cultivation if the concentration of dissolved CO2 in equilibrium with the gas phase CO2 vol% is greater than Ccrit and the prevailing CO2 mass-transfer rate prevents the dissolved CO2 to fall below Ccrit. As the mass-transfer rate of CO2 from gas to culture which replenishes depleted CO2 is controlled by CO2 vol% and volumetric CO2 mass-transfer coefficient (k L a)C, the optimized CO2 vol% should be specific for the prevailing mass-transfer. Hence, a suboptimal CO2 vol% may provide CO2-replete condition in a relatively enhanced mass-transfer condition. On the other hand, since the cost of CO2 in large-scale algal culture is substantial, using an arbitrary high CO2 vol% will be uneconomical if a relatively lower fraction of CO2 can maintain the same CO2-replete condition (Chisti 2013; Tapie and Bernard 1988). Moreover, there is another common practice which needs reassessment. Since pH changes with the dissolved CO2, it is considered as an indicator of dissolved CO2 level in microalgal culture and is usually monitored to identify any significant depletion of CO2 with growth. However, assimilation of nitrogen source also changes the pH of microalgal culture. Therefore, the assessment of CO2 level in microalgal culture by pH-monitoring is not appropriate.
Realizing the need to ensure CO2-replete condition in microalgal photoautotrophic cultures and the challenges in determination of Ccrit, the objective of the present study is to develop a scheme to determine Ccrit and SCUR using DO measurements instead of dissolved CO2, and to estimate CO2-saturated growth from species-specific parameters (Ccrit, SCUR) and operating conditions (CO2 vol%, CO2 mass-transfer) to determine CO2 sufficiency in a photobioreactor culture. CO2 enrichment and mass-transfer required to keep microalgal cells CO2-saturated throughout desired level of growth in photobioreactor can be, therefore, estimated. The green microalga Chlorella vulgaris has been used in the study to demonstrate the proposed methodology for estimation of Ccrit and SCUR, and to validate the estimates of CO2-saturated growth.
Materials and methods
Strain and cultivation conditions
Chlorella vulgaris (211/11B) was purchased from Culture Collection of Algae and Protozoa (CCAP), Scottish Marine Institute, United Kingdom. Cells were grown in Bold’s Basal Medium (BBM) with twofold iron content to prevent iron limitation. Incubation temperature was 25 ± 1 °C and customized white LED light sources of 200 W with pulse width modulation (PWM) controlled brightness were used to provide the required photosynthetic photon flux density (PPFD, µmol/m2/s) to microalgal cultures.
Estimation of CO2-saturated growth
Equation (1) can be used to estimate CO2-saturated growth (x c sat), if Ccrit and SCUR for the microalga (k L a)C and the equilibrium CO2 concentration (C*) in the growth medium at the operating CO2 vol% are known:
Henry’s law (Eq. 2) can be used to approximate C*, since the growth medium is like water:
where \(m_{{{\text{co}}_{ 2 } , {\text{gas}}}}\) is the mole fraction of CO2 in the gas phase, P T is total gas pressure, and \({\text{H}}_{{{\text{CO}}_{ 2} }}\) is Henry’s constant for CO2 which is a function of temperature (\({\text{H}}_{{{\text{CO}}_{ 2} }}\) in pure water at 25 °C is 29.41 L atm/mol). Determining other parameters of Eq. (1) without online measurement of dissolved CO2 is challenging. Relationship (Eq. 3) proposed by Fair (1967) can be used to estimate (k L a)C from experimentally measurable (k L a)O (Babcock et al. 2002; Boogerd et al. 1990):
where \(D^{{{\text{CO}}_{ 2} }}\) and \(D^{{{\text{O}}_{ 2} }}\) are the molecular diffusivities of CO2 and O2 in water, respectively. Diffusivities are temperature dependent and their values at 25 °C are substituted in Eq. (3). SCUR can be estimated theoretically from Eq. (4) if specific O2 production rate (SOPR) and photosynthetic quotient (PQ) are known:
SOPR can be calculated from online measurement of DO (described in “Analytical methods”), whereas PQ (moles of O2 produced per unit mole of CO2 assimilated) can be estimated from the stoichiometric equation of growth (Eq. 5):
where z, the stoichiometric coefficient of O2, needs to be evaluated as it is the measure of PQ. On solving H, O, and N balance equations, z (or PQ) can be completely written in terms of p, q, and r (Eq. 6) which can be easily determined from C, H, O, and N mass fractions of the biomass:
Typically, SOPR increases with the dissolved CO2 concentration and levels off at Ccrit. Moreover, Ccrit and its corresponding SOPR increases with the intensity of light available for photosynthesis, and are maximal at saturating intensity. Substituting maximum values of Ccrit and SOPR in Eqs. (1) and (4) makes x c sat minimal to represent minimum biomass which can be produced without CO2 limitation and maximum biomass which can be produced in CO2- and light-saturated culture. Moreover, since the biomass taken for measurement of SOPR in our experiments was grown in nutrient-replete condition, PQ is also minimal. Therefore, precisely, x c sat estimates the maximum biomass possible in CO2-, light-, and nutrient-replete culture. In general, as the cell density increases inside photobioreactor, light gets attenuated and the nutrient levels, particularly nitrogen began to fall gradually resulting in accumulation of lipid or carbohydrates, thereby changing biomass composition. These changes decrease the values of Ccrit and SOPR and increase the value of PQ (Eriksen et al. 2007). Consequently, the actual biomass produced under CO2 saturation will be greater than x c sat when light intensity and nutrient concentration drop below their saturation level.
Experimental setups
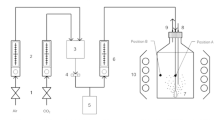
For the measurement of SOPR, a photosynthetic activity measurement system has been designed which minimizes light attenuation by hanging a low depth culture over the light source (Fig. 1a). It consists of two borosilicate transparent glass units, viz., lid and hanging chamber, which are easy to assemble and clean. The lid has three glass joints—a centre joint for glass stirrer and two side joints for oxygen and pH electrodes. The gaps between the glass joints and electrodes or stirrer were sealed with the customized PTFE fittings to provide containment during experiment. A glass paddle stirrer was attached to a speed controlled 12 V DC geared motor overhead for mixing the culture. The hanging chamber is essentially a Petri dish of depth less than 10 mm and fits upright inside the lid cavity. The gap between the lid and hanging chamber was sealed with a silicone gasket. The whole apparatus was placed over the LED light source. Fans were installed near the light source to dissipate heat.
Experiments to validate the accuracy of calculated x c sat were conducted in tube reactors. These reactors were essentially 60 mL glass culture tubes (25 mm outer diameter and 200 mm length) with screw caps bored to pass through silicone air tubes for culture aeration and exhaust gas removal. The gaps between bores and tubes were sealed with silicone sealant. Rotameter was installed to measure and control gas flow to the reactor. Air filters were installed in the inlet and exhaust gas lines. LED light was mounted behind the tube reactors to illuminate cultures. Cooling fans were installed near the reactors to prevent heat accumulation.
Experimental design
Two sets of experiments were performed in the photosynthetic activity measurement system to determine the maximum values of Ccrit and SOPR. In the first set, saturating CO2 level was determined at a supposed saturating/supersaturating intensity by measuring SOPR at different dissolved CO2 concentrations obtained by sparging medium with air or CO2-enriched air (0.14, 0.48, 1.09, 2.12, 3.07, 3.95, and 10.08% v/v) before inoculation. The experiment will provide maximum SOPR and Ccrit at the saturating intensity. The second set of experiments was performed to confirm the saturating intensity supposed in the first set. At the dissolved CO2 level greater than the maximum Ccrit, SOPR should increase with the light intensity and must level off at saturating intensity. SOPR was measured at different light intensities (100–600 µmol/m2/s), at the dissolved CO2 level much higher than the determined Ccrit. Inoculums were prepared in 250 mL Borosil glass bottle sparged with filtered sterilized air/CO2-enriched air, depending on the requirements. Light intensity and CO2 vol% were the same as supposed to be used in the experiment. Cells were harvested for inoculation at low cell density (OD750 between 0.2 and 0.3) to prevent any undesirable change in biomass composition due to nutrient limitation which can affect CO2-uptake and O2-production rates. Prior to inoculation inside laminar hood, the autoclaved medium (without iron) was sparged with sterilized air/CO2-enriched air for at least 10 min to achieve CO2 equilibrium. Under aseptic conditions, a 200 mL of the sparged medium was taken out for titration to quantify dissolved CO2 and a 100 mL, supplemented with autoclaved iron stock, was inoculated with cells harvested from the inoculum to obtain OD750 of ca. 0.05 to prevent light attenuation. A 50 mL of the culture was poured into the sterilized hanging chamber of the photosynthetic activity measurement system and the rest 50 mL was used to determine the dry cell weight (DCW), as described in “Analytical methods”, to calculate biomass titer of the culture. Electrodes and stirrer were inserted into their respective openings on the sterilized lid, and the hanging chamber with the culture was carefully fitted inside the lid cavity with the silicone gasket. A thin needle connected to gas tube was inserted inside from the silicon gasket to flush the air trapped inside the system with the same CO2-enriched air which was used to sparge the growth medium before inoculation. The whole assembly was placed over the LED light source in a dark room (Fig. 1b) and the changes in DO were recorded to determine SOPR as described in the “Analytical methods”. All experiments were performed in triplicate.
In validation experiments, 20 mL cultures were grown in illuminated tube reactors with CO2-enriched air sparging. Two air flow rates (20 and 60 mL/min) of the CO2-enriched air were used separately to vary the (k L a)O. Specific growth rate of the culture was determined after 12 h incubation using Eq. (7).
Analytical methods
PPFD was measured by Quantum sensor (LI-COR LI-190SB, USA). Dissolved CO2 concentration of the medium was determined by the titrimetric method for free carbon dioxide (APHA 1998) immediately after sparging medium (without iron) with CO2-enriched air. A 200 mL of the sparged medium with added phenolphthalein was titrated with N/44 NaOH till the end point, indicated by pink colour. Iron stock was sterilized separately to prevent its interference in titration and was added to the remaining portion of the sparged medium for culturing cells. In addition, medium pH was maintained at 7 before sterilization to prevent the interference of constituent acids during titration with NaOH. Elemental (CHON) composition of biomass was determined by element analyzer Vario EL III (Elementar Analysensysteme, Hanau, Germany). DO and pH electrodes (Easy sense O2 21, Easy sense pH 31, Mettler Toledo, India) were installed to a transmitter (M200 Mettler Toledo India). DO electrode was calibrated in air-saturated distilled water at 25 °C (8.69 mg O2/L at equilibrium) and pH electrode was calibrated with pH buffers of pH 4, 7, and 10. Exhaust gas analyzer (EGAS-L, Sartorius BBI Systems GmbH) was used to determine the CO2 vol% of the CO2-enriched air.
DCW was determined by centrifuging (10 min at 10,000 rpm) culture in a pre-weighed dry centrifuge tube followed by drying the washed pallet at 80 °C for 24 h. SOPR was calculated by dividing the Oxygen Production Rate (OPR, mg O2/L/h) with the biomass titer (mg DCW/L). OPR (mg O2/L/h) was obtained from the slope of the linear portion of the DO curve (generally observed after an initial short acceleration phase) determined by linear regression. The dynamic gassing-out method was used to measure (k L a)O of the tube reactor under the experimental conditions. Specific growth rate (µ) was determined in the validation experiments according to the following equation:
where x 0 and x f are the biomass titers (mg/L) at the initial and after t h, respectively.
Results and discussion
The first set of experiments in the photosynthetic activity measurement system, to determine Ccrit, were performed at 400 µmol/m2/s, since photosynthesis in C. vulgaris has been found to peak at intensities below or about 400 µmol/m2/s (Lloyd et al. 1977). As expected, pH of the medium decreased with the increase in CO2 level (Fig. 2). Since it was observed in a separate set of shake flask experiments that optimum pH for growth of the microalga is 6 and 7 (Fig. S1), pH variations between 6 and 7 arising due to varying degrees of CO2 enrichment will not affect SOPR. All the experiments were initiated with an initial culture OD750 of ca. 0.05 (corresponds to a biomass titer of ca. 30 mg DCW/L). Low biomass titer and low culture depth in the direction of propagation of light assure non-shading condition and the light intensity is virtually the same throughout the culture volume—optically thin culture. We ran each experiment for 20 min. All DO curves were linear following an initial short phase of 2–3 min (Fig. S2). In each case, slope of the DO curve (measure of OPR) between 4 and 14 min has been used to calculate SOPR. During this period, change in pH was negligible and oxygen accumulation (maximum 9.6 mg/L) was insignificant for the inhibition of photosynthesis. Maximum SOPR is 0.142 mg O2/mg DCW/h and the value of Ccrit is taken as 7 mg/L, since it is apparent from Fig. 2 that Ccrit lies between 2 and 7 mg/L dissolved CO2. Similar levels of Ccrit were earlier reported for Chlorella (Markl et al.1985; Warburg 1919). Dissolved CO2 concentration determined by titration was lower than the value obtained from Henry’s Law in each case; however, the difference is not significant in any case (maximum difference is 6.4 mg/L for the titration value of 144.4 mg/L). Therefore, Henry’s law can be used to estimate dissolved CO2 instead of performing titration.
To verify the assumption that 400 µmol/m2/s is the saturating/supersaturating intensity, the second set experiments were performed at a high saturating CO2 level of 30.5 mg/L (Fig. 3) (obtained by sparging ca. 2% v/v CO2-enriched air). The same trend in the DO curves was observed as in the first set of experiments—an initial short acceleration phase followed by a linear region (Fig. S3). SOPR increased with the light intensity and levelled off at 300 µmol/m2/s reaching the same maximum value of 0.142 mg O2/mg DCW/h (Fig. 3). This verifies that 400 µmol/m2/s is the supersaturating intensity. Elemental formula of the strain (CH1.649 O0.373 N0.127) is determined from the composition of the biomass (54% C, 7.42% H, 26.05% O, and 8% N) grown in inoculums with low cell density and dissolved CO2 levels above Ccrit to prevent changes in biomass composition due to CO2 limitation. The value of PQ calculated from Eq. (6) is 0.3845 and the value of SCUR obtained by substituting SOPR (0.142 mg O2/mg DCW/h) and PQ in Eq. (4) is 0.5078 mg CO2/mg DCW/h. From the values of Ccrit and SCUR, x c sat can be determined from Eq. (1) for the given CO2 fraction of the sparged air and (k L a)C of the photobioreactor determined from Eq. (3).
In validation experiments, cultures (OD750 ca. 0.05) were grown in tube reactors (optically thin cultures) at supersaturating intensity (500 µmol/m2/s) for 12 h to prevent light and nutrient limitation. Results of the four experiments performed are given in Table 1. It can be noted that all the cultures with final biomass titer x f < x c sat grew at an specific growth rate of ca. 2.2/day, whereas the culture with x f > x c sat grew at comparatively lower rate (2.02/day). The growth is limited by CO2 when x f > x c sat under light and nutrient-replete condition. Decrease in the specific growth rate when x f > x c sat is a manifestation of CO2-limited growth. Although the difference of this scale is generally not considered, but it is significant in this case, since the photoautotrophic algal growth is too slow to manifest prominent change in specific growth rate due to changes in CO2 levels. Moreover, very low standard deviations in all the four cases (Table 1) indicate the precision in measurement and support the significance of the observed difference in specific growth rates. The results validate the estimated x c sat under different operational conditions.
Based on the measured Ccrit and SCUR, a contour map of x c sat for typical range of (k L a)O and CO2 volume fractions was prepared (Fig. 4). C* was calculated from Henry’s law (Eq. 2) at atmospheric pressure and 25 °C. Combinations of CO2 vol% and (k L a)O required to sustain CO2-replete growth of C. vulgaris up to high biomass titers can be figured out directly from the contour map. It is evident from the contour plot that even at low CO2 vol%, relatively high CO2-saturated growth is feasible in stirred tank reactors ((k L a)O 100–300/h) than in bubble columns ((k L a)O < 100/h). In addition, in stirred tank reactors with (k L a)O > 100/h, 5–10% CO2 can support biomass titer too high to achieve practically. However, irrespective of the type of photobioreactor used for cultivation, relatively low quantity of algal biomass is generally produced in photoautotrophic batch cultivation as the growth is limited by several factors. In addition to inadequate CO2-enrichment and mass-transfer, the microalgal growth is generally limited by light attenuation, nitrogen depletion, and oxygen accumulation at inhibitory levels. It is, therefore, essential to determine the CO2 vol% just required to provide CO2 sufficiency throughout the cultivation. Under the prevailing mass-transfer condition inside the photobioreactor, a minimum CO2 vol% required to support a target CO2-saturated growth should be first calculated. If the biomass produced at calculated CO2 vol% is less than the target, then the growth is limited by factors other than CO2 and consequently a relatively lower CO2 vol% can be selected, such that the cells are limited by other factors before CO2 could limit the growth. Lowering CO2 vol% is economically favourable, especially at large scale. However, high CO2 concentrations may be useful when oxygen accumulates to inhibitory levels.
References
APHA (1998) Standard methods for the examination of water and wastewater, 20th edn. American Public Health Association, Washington, DC
Babcock RW, Malda J, Radway JC (2002) Hydrodynamics and mass transfer in a tubular airlift photobioreactor. J Appl Phycol 14:169–184
Boogerd FC, Bos P, Kuenen JG, Heijnen JJ, van der Lans RG (1990) Oxygen and carbon dioxide mass transfer and the aerobic, autotrophic cultivation of moderate and extreme thermophiles: a case study related to the microbial desulfurization of coal. Biotechnol Bioeng 35:1111–1119
Brindley C, Fernández FG, Fernández-Sevilla JM (2011) Analysis of light regime in continuous light distributions in photobioreactors. Bioresour Technol 102:3138–3148
Chisti Y (2013) Constraints to commercialization of algal fuels. J Biotechnol 167:201–214
Eriksen NT (2008) The technology of microalgal culturing. Biotechnol Lett 30:1525–1536
Eriksen NT, Riisgård FK, Gunther WS, Lønsmann-Iversen JJ (2007) On-line estimation of O2 production, CO2 uptake, and growth kinetics of microalgal cultures in a gas-tight photobioreactor. J Appl Phycol 19:161–174
Fair JR (1967) Designing gas-sparged reactors. Chem Eng 74:67–74
Fernández FG, Camacho FG, Pérez JA, Sevilla JM, Grima EM (1997) A model for light distribution and average solar irradiance inside outdoor tubular photobioreactors for the microalgal mass culture. Biotechnol Bioeng 55:701–714
Lloyd ND, Canvin DT, Culver DA (1977) Photo-synthesis and photorespiration in algae. Plant Physiol 59:936–940
Markl H, Mather M (1985) Mixing and aeration of shallow open ponds. Arch Hydrobiol Beih 20:85–93
Merchuk JC, Garcia-Camacho F, Molina-Grima E (2007) Photobioreactor design and fluid dynamics. Chem Biochem Eng Q 21:345–355
Molina-Grima E, Acién-Fernández FG, Garcia-Camacho F, Chisti Y (1999) Photobioreactors: light regime, mass transfer, and scaleup. J Biotechnol 70:231–247
Olivieri G, Salatino P, Marzocchella A (2014) Advances in photobioreactors for intensive microalgal production: configurations, operating strategies and applications. J Chem Technol Biotechnol 89:178–195
Pottier L, Pruvost J, Deremetz J, Cornet JF, Legrand J, Dussap CG (2005) A fully predictive model for one-dimensional light attenuation by Chlamydomonas reinhardtii in a torus photobioreactor. Biotechnol Bioeng 91:569–582
Pruvost J, Cornet JF, Legrand J (2008) Hydrodynamics influence on light conversion in photobioreactors: an energetically consistent analysis. Chem Eng Sci 63:3679–3694
Tapie P, Bernard A (1988) Microalgae production: technical and economic evaluations. Biotechnol Bioeng 32:873–885
Ugwu CU, Aoyagi H, Uchiyama H (2008) Photobioreactors for mass cultivation of algae. Bioresour Technol 99:4021–4028
Warburg O (1919) Uber die geschwindigkeit der photochemischen kohlensaurezersetzung in lebenden zellen. Biochem Z 100:230–270
Yun YS, Park JM (2003) Kinetic modeling of the light-dependent photosynthetic activity of the green microalga Chlorella vulgaris. Biotechnol Bioeng 83:303–311
Acknowledgements
Yuvraj greatly acknowledges the support from his parents and other family members. Authors are thankful to Central Instrumentation Facility (CIF), BIT, Mesra, Ranchi for performing the elemental analysis of the algal biomass.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to declare.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made.
The images or other third party material in this article are included in the article’s Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder.
To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yuvraj, Padmanabhan, P. Technical insight on the requirements for CO2-saturated growth of microalgae in photobioreactors. 3 Biotech 7, 119 (2017). https://doi.org/10.1007/s13205-017-0778-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s13205-017-0778-6