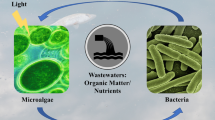
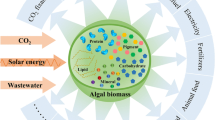
Abstract
Microalgae, as the most promising raw material for biodiesel, can absorb and transform N, P, and organic matter in wastewater into cell components such as oil, carbohydrate, and protein. The cultivation of microalgae in wastewater provides the possibility to realize harmless treatment and resource utilization of wastewater and reduce the cost of microalgal culture. Whether in a single microalgal culture process or wastewater system, there always be a large number of bacteria interacting with microalgae, forming a complex microecological system. Although microalgal–bacterial coculture system is feasible features for the higher biomass production and better nutrient adaptability from wastewater, the precise understanding of interaction and coexistence in water bodies is still unclear. Some major challenges are to develop sustainability in the microalgal–bacterial coculture system due to the incomplete knowledge about the mechanism of communication, algal growth promotion, and effect of microalgae on the indigenous bacteria in wastewater. In the present review, nutritional interaction and signal transduction, two main interaction models of algae and bacteria, and the effect of synergism of these two models on microalgal system are summarized. Moreover, the impact of algal–bacterial coculture on algal growth and removal of pollutants in wastewater treatment process was expounded systematically to develop highly efficient consortium systems by covering the sustainability limitations. This work would provide guidance for the establishment of efficient algal–bacterial symbiosis system in the wastewater with complex microecology and nutrient environment to improve the accumulation of microalgal biomass and reduce the cost of microalgal culture.




Similar content being viewed by others
References
Han Y, Wu H, Geng Z, Zhu Q (2020) Review: energy efficiency evaluation of complex petrochemical industries. Energy. 203:117893. https://doi.org/10.1016/j.energy.2020.117893
Jia H, Yuan Q (2016) Removal of nitrogen from wastewater using microalgae and microalgae-bacteria consortia. Cogent Environ Sci 2. https://doi.org/10.1080/23311843.2016.1275089
Stark JS, Smith J, King CK, Lindsay M, Stark S, Palmer AS, Snape I, Bridgen P, Riddle M (2015) Physical, chemical, biological and ecotoxicological properties of wastewater discharged from Davis Station, Antarctica. Cold Reg Sci Technol 113:52–62. https://doi.org/10.1016/j.coldregions.2015.02.006
Fan J, Chen Y, Zhang TC, Ji B, Cao L (2020) Performance of Chlorella sorokiniana-activated sludge consortium treating wastewater under light-limited heterotrophic condition. Chem Eng J 382:122799. https://doi.org/10.1016/j.cej.2019.122799
Kumar PK, Krishna SV, Naidu SS, Verma K, Bhagawan D, Himabindu V (2019) Biomass production from microalgae Chlorella grown in sewage, kitchen wastewater using industrial CO2 emissions: comparative study. Carbon Resour Convers 2:126–133. https://doi.org/10.1016/j.crcon.2019.06.002
Delgadillo-Mirquez L, Lopes F, Taidi B, Pareau D (2016) Nitrogen and phosphate removal from wastewater with a mixed microalgae and bacteria culture. Biotechnol Rep 11:18–26. https://doi.org/10.1016/j.btre.2016.04.003
Wu Y, Hu H, Yu Y et al (2014) Microalgal species for sustainable biomass/lipid production using wastewater as resource: a review. Renew Sust Energ Rev 33:675–688. https://doi.org/10.1016/j.rser.2014.02.026
Choi KJ, Han TH, Yoo G, Cho MH, Hwang SJ (2018) Co-culture consortium of Scenedesmus dimorphus and nitrifiers enhances the removal of nitrogen and phosphorus from artificial wastewater. KSCE J Civ Eng 22:3215–3221. https://doi.org/10.1007/s12205-017-0730-7
Mohd-Sahib AA, Lim JW, Lam MK, Uemura Y, Isa MH, Ho CD, Kutty SRM, Wong CY, Rosli SS (2017) Lipid for biodiesel production from attached growth Chlorella vulgaris biomass cultivating in fluidized bed bioreactor packed with polyurethane foam material. Bioresour Technol 239:127–136. https://doi.org/10.1016/j.biortech.2017.04.118
Chowdhury H, Loganathan B, Mustary I et al (2019) Co-cultivation of activated sludge and microalgae for the simultaneous enhancements of nitrogen-rich wastewater bioremediation and lipid production. Sustain. https://doi.org/10.1016/B978-0-12-815162-4.00012-4
Bhatia SK, Mehariya S, Bhatia RK, Kumar M, Pugazhendhi A, Awasthi MK, Atabani AE, Kumar G, Kim W, Seo SO, Yang YH (2020) Wastewater based microalgal biorefinery for bioenergy production: progress and challenges. Sci Total Environ 751:141599. https://doi.org/10.1016/j.scitotenv.2020.141599
Krug L, Morauf C, Donat C, Müller H, Cernava T, Berg G (2020) Plant growth-promoting Methylobacteria selectively increase the biomass of biotechnologically relevant microalgae. Front Microbiol 11. https://doi.org/10.3389/fmicb.2020.00427
Huo S, Kong M, Zhu F, Qian J, Huang D, Chen P, Ruan R (2020) Co-culture of Chlorella and wastewater-borne bacteria in vinegar production wastewater: enhancement of nutrients removal and influence of algal biomass generation. Algal Res 45:101744. https://doi.org/10.1016/j.algal.2019.101744
Daverey A, Pandey D, Verma P, Verma S, Shah V, Dutta K, Arunachalam K (2019) Recent advances in energy efficient biological treatment of municipal wastewater. Bioresour Technol Rep 7:100252. https://doi.org/10.1016/j.biteb.2019.100252
Mujtaba G, Rizwan M, Kim G, Lee K (2018) Removal of nutrients and COD through co-culturing activated sludge. Chem Eng J 343:155–162. https://doi.org/10.1016/j.cej.2018.03.007
Russel M, Meixue Q, Alam A et al (2019) Investigating the potentiality of Scenedesmus obliquus and Acinetobacter pittii partnership system and their effects on nutrients removal from synthetic domestic wastewater. Bioresour Technol 299:122571. https://doi.org/10.1016/j.biortech.2019.122571
Ji X, Jiang M, Zhang J, Jiang X, Zheng Z (2018) The interactions of algae-bacteria symbiotic system and its effects on nutrients removal from synthetic wastewater. Bioresour Technol 247:44–50. https://doi.org/10.1016/j.biortech.2017.09.074
Kouzuma A, Watanabe K (2015) Exploring the potential of algae/bacteria interactions. Curr Opin Biotechnol 33:125–129. https://doi.org/10.1016/j.copbio.2015.02.007
Xue L, Shang H, Ma P, Wang X, He X, Niu J, Wu J (2018) Analysis of growth and lipid production characteristics of Chlorella vulgaris in artificially constructed consortia with symbiotic bacteria. J Basic Microbiol 58:358–367. https://doi.org/10.1002/jobm.201700594
Ramanan R, Kim BH, Cho DH, Oh HM, Kim HS (2016) Algae-bacteria interactions: evolution, ecology and emerging applications. Biotechnol Adv 34:14–29. https://doi.org/10.1016/j.biotechadv.2015.12.003
Fuentes JL, Garbayo I, Cuaresma M, Montero Z, González-del-Valle M, Vílchez C (2016) Impact of microalgae-bacteria interactions on the production of algal biomass and associated compounds. Mar Drugs 14. https://doi.org/10.3390/md14050100
Zhang J, Tian Y, Zuo W et al (2016) Effect of aeration rate on performance and stability of algal-bacterial symbiosis system to treat domestic wastewater in sequencing batch reactors. Bioresour Technol 222:156–164. https://doi.org/10.1016/j.biortech.2016.09.123
Sutherland DL, Park J, Heubeck S, Ralph PJ, Craggs RJ (2020) Size matters-microalgae production and nutrient removal in wastewater treatment high rate algal ponds of three different sizes. Algal Res 45:101734. https://doi.org/10.1016/j.algal.2019.101734
Wollmann F, Walther T, Dietze S et al (2019) Microalgae wastewater treatment: biological and technological approaches. Eng Life Sci 19:860–871. https://doi.org/10.1002/elsc.201900071
Mohd Udaiyappan AF, Hasan HA, Takriff MS, Abdullah SRS, Maeda T, Mustapha NA, Mohd Yasin NH, Nazashida Mohd Hakimi NI (2020) Microalgae-bacteria interaction in palm oil mill effluent treatment. J Water Process Eng 35:101203. https://doi.org/10.1016/j.jwpe.2020.101203
Ramanan R, Kang Z, Kim BH, Cho DH, Jin L, Oh HM, Kim HS (2015) Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. Algal Res 8:140–144. https://doi.org/10.1016/j.algal.2015.02.003
Randrianarison G, Ashraf MA (2017) Microalgae: a potential plant for energy production. Geol Ecol Landsc 1:104–120. https://doi.org/10.1080/24749508.2017.1332853
Xiao-qian Z, Zhe-yu LI, Xiao-yan Z et al (2017) Advance in bacteria chemotaxis on microfluidic devices. Chin J Anal Chem 45:1734–1744. https://doi.org/10.1016/S1872-2040(17)61050-8
Cooper MB, Smith AG (2015) Exploring mutualistic interactions between microalgae and bacteria in the omics age. Curr Opin Plant Biol 26:147–153. https://doi.org/10.1016/j.pbi.2015.07.003
Hong J, Luo Y, Mou M, Fu J, Zhang Y, Xue W, Xie T, Tao L, Lou Y, Zhu F (2019) Convolutional neural network-based annotation of bacterial type IV secretion system effectors with enhanced accuracy and reduced false discovery. Brief Bioinform 21:1825–1836. https://doi.org/10.1093/bib/bbz120
Zhao R, Chen G, Liu L, Zhang W, Sun Y, Li B, Wang G (2020) Bacterial foraging facilitates aggregation of Chlamydomonas microsphaera in an organic carbon source-limited aquatic. Environ Pollut 259:113924. https://doi.org/10.1016/j.envpol.2020.113924
Khan MI, Shin JH, Kim JD (2018) The promising future of microalgae: current status, challenges, and optimization of a sustainable and renewable industry for biofuels, feed, and other products. Microb Cell Factories 17:36. https://doi.org/10.1186/s12934-018-0879-x
Tandon P, Jin Q, Huang L (2017) A promising approach to enhance microalgae productivity by exogenous supply of vitamins. Microb Cell Factories 16:219. https://doi.org/10.1186/s12934-017-0834-2
Lépinay A, Turpin V, Mondeguer F, Grandet-marchant Q (2018) First insight on interactions between bacteria and the marine diatom Haslea ostrearia: algal growth and metabolomic fingerprinting. Algal Res 31:395–405. https://doi.org/10.1016/j.algal.2018.02.023
Chi W, Zheng L, He C et al (2017) Quorum sensing of microalgae associated marine Ponticoccus sp. PD-2 and its algicidal function regulation. AMB Express. https://doi.org/10.1186/s13568-017-0357-6
Han S, Jeon MS, Heo YM et al (2020) Effect of Pseudoalteromonas sp. MEBiC 03485 on biomass production and sulfated polysaccharide biosynthesis in Porphyridium cruentum UTEX 161. Bioresour Technol. https://doi.org/10.1016/j.biortech.2020.122791
Paddock MB, Fernández-Bayo JD, VanderGheynst JS (2020) The effect of the microalgae-bacteria microbiome on wastewater treatment and biomass production. Appl Microbiol Biotechnol 104:893–905. https://doi.org/10.1007/s00253-019-10246-x
Hai C, Vu T, Lee H et al (2017) Axenic cultures for microalgal biotechnology: establishment, assessment, maintenance, and applications. Biotechnol Adv. https://doi.org/10.1016/j.biotechadv.2017.12.018
Yao S, Lyu S, An Y, Lu J, Gjermansen C, Schramm A (2019) Microalgae-bacteria symbiosis in microalgal growth and biofuel production: a review. J Appl Microbiol 126:359–368. https://doi.org/10.1111/jam.14095
Palacios OA, Lopez BR, Bashan Y, de-Bashan LE (2019) Early changes in nutritional conditions affect formation of synthetic mutualism between Chlorella sorokiniana and the bacterium Azospirillum brasilense. Microb Ecol 77:980–992. https://doi.org/10.1007/s00248-018-1282-1
Trögl SRAPBJ (2020) New perspectives on the bioremediation of endocrine disrupting compounds from wastewater using algae-, bacteria- and fungi-based technologies. Int J Environ Sci Technol. https://doi.org/10.1007/s13762-020-02691-3
Lam TP, Lee T, Chen C, Chang J (2017) Strategies to control biological contaminants during microalgal cultivation in open ponds. Bioresour Technol 252:180–187. https://doi.org/10.1016/j.biortech.2017.12.088
Guo Z, Tong YW (2014) The interactions between Chlorella vulgaris and algal symbiotic bacteria under photoautotrophic and photoheterotrophic conditions. J Appl Phycol 26:1483–1492. https://doi.org/10.1007/s10811-013-0186-1
Hoseinifar SH, Sun Y, Wang A et al (2018) Probiotics as means of diseases control in aquaculture, a review of current knowledge and future perspectives. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.02429
Seymour JR, Amin SA, Raina JB, Stocker R (2017) Zooming in on the phycosphere: the ecological interface for phytoplankton-bacteria relationships. Nat Microbiol 2. https://doi.org/10.1038/nmicrobiol.2017.65
Li Q, Ren Y, Fu X (2019) Inter-kingdom signaling between gut microbiota and their host. Cell Mol Life Sci 76:2383–2389. https://doi.org/10.1007/s00018-019-03076-7
Padmaperuma G, Kapoore RV, Gilmour DJ, Group F (2017) Microbial consortia: a critical look at microalgae co-cultures for enhanced biomanufacturing. Crit Rev Biotechnol 38:690–703. https://doi.org/10.1080/07388551.2017.1390728
Pacheco D, Rocha AC, Pereira L, Verdelhos T (2020) Microalgae water bioremediation: trends and hot topics. Appl Sci. https://doi.org/10.3390/app10051886
Wei Z, Huang S, Zhang Y (2017) Characterization of extracellular polymeric substances produced during nitrate removal by a thermophilic bacterium Chelatococcus daeguensis TAD1 in batch cultures. RSC Adv 7:44265–44271. https://doi.org/10.1039/C7RA08147B
Eigemann F, Hilt S, Salka I, Grossart H (2012) Bacterial community composition associated with freshwater algae: species specificity vs. dependency on environmental conditions and source community. FEMS Microbiol Ecol. https://doi.org/10.1111/1574-6941.12022
Ryu BG, Kim W, Nam K, Kim S, Lee B, Park MS, Yang JW (2015) A comprehensive study on algal-bacterial communities shift during thiocyanate degradation in a microalga-mediated process. Bioresour Technol 191:496–504. https://doi.org/10.1016/j.biortech.2015.03.136
Dao GH, Wu GX, Wang XX et al (2018) Enhanced microalgae growth through stimulated secretion of indole acetic acid by symbiotic bacteria. Algal Res 33:345–351. https://doi.org/10.1016/j.algal.2018.06.006
Yang W, Zheng Z, Lu K, Zheng C, du Y, Wang J, Zhu J (2019) Manipulating the phytoplankton community has the potential to create a stable bacterioplankton community in a shrimp rearing environment. Aquaculture. 520:734789. https://doi.org/10.1016/j.aquaculture.2019.734789
Sandhya SV, Sandeep KP, Vijayan KK (2020) In vivo evaluation of microbial cocktail of microalgae-associated bacteria in larval rearing from zoea I to mysis I of the Indian white shrimp, Penaeus indicus. J Appl Phycol. https://doi.org/10.1007/s10811-020-02230-0
Dobretsov S, Rittschof D (2020) Love at first taste: induction of larval settlement by marine microbes. Int J Mol Sci 21. https://doi.org/10.3390/ijms21030731
Nie X, Mubashar M, Zhang S, Qin Y, Zhang X (2020) Current progress, challenges and perspectives in microalgae-based nutrient removal for aquaculture waste: a comprehensive review. J Clean Prod 277:124209. https://doi.org/10.1016/j.jclepro.2020.124209
Zhang Z, Chen M, Li J, Zhao B, Wang L (2019) Significance of transparent exopolymer particles derived from aquatic algae in membrane fouling. Arab J Chem 13:4577–4585. https://doi.org/10.1016/j.arabjc.2019.10.004
Shahid A, Malik S, Zhu H, Xu J, Nawaz MZ, Nawaz S, Asraful Alam M, Mehmood MA (2019) Cultivating microalgae in wastewater for biomass production, pollutant removal, and atmospheric carbon mitigation; a review. Sci Total Environ 704:135303. https://doi.org/10.1016/j.scitotenv.2019.135303
Sciences M (2019) Metabolic pathway analysis of nitrogen and phosphorus uptake by the consortium between C. vulgaris and P. aeruginosa. Int J Mol Sci. https://doi.org/10.3390/ijms20081978
Cui Y, Chun S, Baek S et al (2020) Unique microbial module regulates the harmful algal bloom (Cochlodinium polykrikoides) and shifts the microbial community along the southern coast of Korea. Sci Total Environ 721:137725. https://doi.org/10.1016/j.scitotenv.2020.137725
Silva L, Ll M, Ivetic S et al (2021) Heterotrophic bacterioplankton responses in coral- and algae-dominated Red Sea reefs show they might benefit from future regime shift. Sci Total Environ 751:141628. https://doi.org/10.1016/j.scitotenv.2020.141628
Xiao R, Zheng Y (2016) Overview of microalgal extracellular polymeric substances (EPS) and their applications. Biotechnol Adv 34:1225–1244. https://doi.org/10.1016/j.biotechadv.2016.08.004
Wang J, Lei Z, Tian C, Liu S, Wang Q, Shimizu K, Zhang Z, Adachi Y, Lee DJ (2020) Ionic response of algal-bacterial granular sludge system during biological phosphorus removal from wastewater. Chemosphere. 264:128534. https://doi.org/10.1016/j.chemosphere.2020.128534
Zhang X, Ye X, Chen L, Zhao H, Shi Q, Xiao Y, Ma L, Hou X, Chen Y, Yang F (2020) Functional role of bloom-forming cyanobacterium Planktothrix in ecologically shaping aquatic environments. Sci Total Environ 710:136314. https://doi.org/10.1016/j.scitotenv.2019.136314
Amin SA, Hmelo LR, Van Tol HM et al (2015) Interaction and signalling between a cosmopolitan phytoplankton and associated bacteria. Nature. 522:98–101. https://doi.org/10.1038/nature14488
Leong WH, Lim JW, Lam MK, Uemura Y, Ho CD, Ho YC (2018) Co-cultivation of activated sludge and microalgae for the simultaneous enhancements of nitrogen-rich wastewater bioremediation and lipid production. J Taiwan Inst Chem Eng 87:216–224. https://doi.org/10.1016/j.jtice.2018.03.038
Gude S, Taga ME (2020) Multi-faceted approaches to discovering and predicting microbial nutritional interactions. Curr Opin Biotechnol 62:58–64. https://doi.org/10.1016/j.copbio.2019.08.005
Schoffman H, Lis H, Shaked Y, Keren N (2016) Iron-nutrient interactions within phytoplankton. Front Plant Sci 7. https://doi.org/10.3389/fpls.2016.01223
Kurth C, Wasmuth I, Wichard T, Pohnert G, Nett M (2019) Algae induce siderophore biosynthesis in the freshwater bacterium Cupriavidus necator H16. BioMetals. 32:77–88. https://doi.org/10.1007/s10534-018-0159-6
Leong WH, Azella Zaine SN, Ho YC, Uemura Y, Lam MK, Khoo KS, Kiatkittipong W, Cheng CK, Show PL, Lim JW (2019) Impact of various microalgal-bacterial populations on municipal wastewater bioremediation and its energy feasibility for lipid-based biofuel production. J Environ Manag 249:109384. https://doi.org/10.1016/j.jenvman.2019.109384
Mukherjee S, Bassler BL (2019) Bacterial quorum sensing in complex and dynamically changing environments. Nat Rev Microbiol 17:371–382. https://doi.org/10.1038/s41579-019-0186-5
Prescott RD, Decho AW (2019) Flexibility and adaptability of quorum sensing in nature. Trends Microbiol 28:436–444. https://doi.org/10.1016/j.tim.2019.12.004
Mangwani N, Dash HR, Chauhan A, Das S (2012) Bacterial quorum sensing: functional features and potential applications in biotechnology. J Mol Microbiol Biotechnol 22:215–227. https://doi.org/10.1159/00034184
Jatt AN, Tang K, Liu J, Zhang Z, Zhang XH (2015) Quorum sensing in marine snow and its possible influence on production of extracellular hydrolytic enzymes in marine snow bacterium Pantoea ananatis B9. FEMS Microbiol Ecol 91:1–13. https://doi.org/10.1093/femsec/fiu030
Singh AA, Singh AK (2020) Bacteria associated with marine macroorganisms as potential source of quorum-sensing antagonists. J Basic Microbiol. https://doi.org/10.1002/jobm.202000231
Zhang B, Li W, Guo Y, Zhang Z, Shi W, Cui F, Lens PNL, Tay JH (2020) Microalgal-bacterial consortia: from interspecies interactions to biotechnological applications. Renew Sust Energ Rev 118:109563. https://doi.org/10.1016/j.rser.2019.109563
Li Q, Gu P, Zhang H et al (2019) Response of submerged macrophytes and leaf biofilms to the decline phase of Microcystis aeruginosa: antioxidant response, ultrastructure, microbial properties, and potential mechanism. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2019.134325
Girard L (2019) Quorum sensing in Vibrio spp.: the complexity of multiple signalling molecules in marine and aquatic environments. Crit Rev Microbiol. https://doi.org/10.1080/1040841X.2019.1624499
Durham BP, Sharma S, Luo H, Smith CB, Amin SA, Bender SJ, Dearth SP, van Mooy BAS, Campagna SR, Kujawinski EB, Armbrust EV, Moran MA (2015) Cryptic carbon and sulfur cycling between surface ocean plankton. Proc Natl Acad Sci U S A 112:453–457. https://doi.org/10.1073/pnas.1413137112
Kazamia E, Czesnick H, Van Nguyen TT et al (2012) Mutualistic interactions between vitamin B12-dependent algae and heterotrophic bacteria. Environ Microbiol 14:1466–1476. https://doi.org/10.1111/j.1462-2920.2012.02733.x
Wang M, Chen S, Zhou W, Yuan W, Wang D (2020) Algal cell lysis by bacteria: a review and comparison to conventional methods. Algal Res 46:101794. https://doi.org/10.1016/j.algal.2020.101794
Lopez BR, Palacios OA, Bashan Y, Hernández-Sandoval FE, de-Bashan LE (2019) Riboflavin and lumichrome exuded by the bacterium Azospirillum brasilense promote growth and changes in metabolites in Chlorella sorokiniana under autotrophic conditions. Algal Res 44:101696. https://doi.org/10.1016/j.algal.2019.101696
Pagnussat LA, Maroniche G, Curatti L, Creus C (2020) Auxin-dependent alleviation of oxidative strecass and growth promotion of Scenedesmus obliquus C1S by Azospirillum brasilense. Algal Res 47:101839. https://doi.org/10.1016/j.algal.2020.101839
Rajapitamahuni S, Bachani P, Sardar RK, Mishra S (2019) Co-cultivation of siderophore-producing bacteria Idiomarina loihiensis RS14 with Chlorella variabilis ATCC 12198, evaluation of micro-algal growth, lipid, and protein content under iron starvation. J Appl Phycol 31:29–39. https://doi.org/10.1007/s10811-018-1591-2
Santos CA, Reis A (2014) Microalgal symbiosis in biotechnology. Appl Microbiol Biotechnol 98:5839–5846. https://doi.org/10.1007/s00253-014-5764-x
Amin SA, Green DH, Hart MC, Ku FC (2009) Photolysis of iron-siderophore chelates promotes bacterial-algal mutualism. Proc Natl Acad Sci 106:17071–17076. https://doi.org/10.1073/pnas.0905512106
Leyva LA, Bashan Y, Mendoza A, Luz E (2014) Accumulation fatty acids of in Chlorella vulgaris under heterotrophic conditions in relation to activity of acetyl-CoA carboxylase, temperature, and co-immobilization with Azospirillum brasilense. Naturwissenschaften. 101:819–830. https://doi.org/10.1007/s00114-014-1223-x
Choix FJ, Luz E, Bashan Y (2012) Enhanced accumulation of starch and total carbohydrates in alginate-immobilized Chlorella spp. induced by Azospirillum brasilense: II. Heterotrophic conditions. Enzym Microb Technol. https://doi.org/10.1016/j.enzmictec.2012.07.012
Zhang C, Li Q, Fu L, Zhou D, Crittenden JC (2018) Quorum sensing molecules in activated sludge could trigger microalgae lipid synthesis. Bioresour Technol 263:576–582. https://doi.org/10.1016/j.biortech.2018.05.045
Laganenka L, Sourjik V (2018) Autoinducer 2-dependent Escherichia coli biofilm formation is enhanced in a dual-species coculture. Appl Environ Microbiol 84. https://doi.org/10.1128/AEM.02638-17
Zhao J, Li X, Hou X et al (2019) Widespread existence of quorum sensing inhibitors in marine bacteria: potential drugs to combat pathogens with novel strategies. Mar Drugs. https://doi.org/10.3390/md17050275
Meschwitz SM, Teasdale ME, Mozzer A, Martin N, Liu J, Forschner-Dancause S, Rowley DC (2019) Antagonism of quorum sensing phenotypes by analogs of the marine bacterial secondary. Mar Drugs 17. https://doi.org/10.3390/md17070389
Hu Z, Qi Y, Zhao L, Chen G (2018) Interactions between microalgae and microorganisms for wastewater remediation and biofuel production. Waste Biomass Valoriz 10:3907–3919. https://doi.org/10.1007/s12649-018-0325-7
Zhou D, Zhang C, Fu L et al (2017) Responses of the microalga Chlorophyta sp. to bacterial quorum sensing molecules (N-acylhomoserine lactones): aromatic protein-induced self-aggregation. Environ Sci Technol. https://doi.org/10.1021/acs.est.7b00355
Natrah FMI, Kenmegne MM, Wiyoto W, Sorgeloos P, Bossier P, Defoirdt T (2011) Effects of micro-algae commonly used in aquaculture on acyl-homoserine lactone quorum sensing. Aquaculture. 317:53–57. https://doi.org/10.1016/j.aquaculture.2011.04.038
Ghimire U, Nandimandalam H, Martinez-guerra E, Gude VG (2019) Wetlands for wastewater treatment. Water Environ Fed 91:1378–1389. https://doi.org/10.1002/wer.1232
Kohlheb N, Van Afferden M, Lara E et al (2020) Assessing the life-cycle sustainability of algae and bacteria-based wastewater treatment systems: high-rate algae pond and sequencing batch reactor. J Environ Manag 264:110459. https://doi.org/10.1016/j.jenvman.2020.110459
Victoria M, Capson-tojo G, Gal A et al (2020) Microalgae-bacteria consortia in high-rate ponds for treating urban wastewater: elucidating the key state indicators under dynamic conditions. J Environ Manag 261:110244. https://doi.org/10.1016/j.jenvman.2020.110244
Loganathan BG, Orsat V, Lefsrud M (2020) Phycoremediation and valorization of synthetic dairy wastewater using microalgal consortia of Chlorella variabilis and Scenedesmus obliquus. Environ Technol 1–14. https://doi.org/10.1080/09593330.2020.1725143
Ji X, Li H, Zhang J, Saiyin H, Zheng Z (2019) The collaborative effect of Chlorella vulgaris-Bacillus licheniformis consortia on the treatment of municipal water. J Hazard Mater 365:483–493. https://doi.org/10.1016/j.jhazmat.2018.11.039
Tait K, White DA, Kimmance SA, Tarran G, Rooks P, Jones M, Llewellyn CA (2019) Characterisation of bacteria from the cultures of a Chlorella strain isolated from textile wastewater and their growth enhancing effects on the axenic cultures of Chlorella vulgaris in low nutrient media. Algal Res 44:101666. https://doi.org/10.1016/j.algal.2019.101666
Mujtaba G, Lee K (2016) Advanced treatment of wastewater using symbiotic co-culture of microalgae and bacteria. Appl Chem Eng. https://doi.org/10.14478/ace.2016.1002
Lee CS, Oh HSHM, Oh HSHM et al (2016) Two-phase photoperiodic cultivation of algal-bacterial consortia for high biomass production and efficient nutrient removal from municipal wastewater. Bioresour Technol 200:867–875. https://doi.org/10.1016/j.biortech.2015.11.007
Ferro L, Colombo M, Posadas E et al (2019) Elucidating the symbiotic interactions between a locally isolated microalga Chlorella vulgaris and its co-occurring bacterium Rhizobium sp. in synthetic municipal wastewater. J Appl Phycol. https://doi.org/10.1007/s10811-019-1741-1
Ho S-H, Chen Y-D, Qu W-Y et al (2019) Algal culture and biofuel production using wastewater. Biofuels Algae. https://doi.org/10.1016/B978-0-444-64192-2.00008-1
Wang S, Liu J, Li C, Chung BM (2018) Efficiency of Nannochloropsis oculata and Bacillus polymyxa symbiotic composite at ammonium and phosphate removal from synthetic wastewater. Environ Technol 40:2494–2503. https://doi.org/10.1080/09593330.2018.1444103
Gonçalves AL, Pires JCM, Simões M (2017) A review on the use of microalgal consortia for wastewater treatment. Algal Res 24:403–415. https://doi.org/10.1016/j.algal.2016.11.008
Zhao X, Zhou Y, Huang S, Qiu D, Schideman L, Chai X, Zhao Y (2014) Characterization of microalgae-bacteria consortium cultured in landfill leachate for carbon fixation and lipid production. Bioresour Technol 156:322–328. https://doi.org/10.1016/j.biortech.2013.12.112
Ren HY, Liu BF, Kong F, Zhao L, Ren N (2015) Hydrogen and lipid production from starch wastewater by co-culture of anaerobic sludge and oleaginous microalgae with simultaneous COD, nitrogen and phosphorus removal. Water Res 85:404–412. https://doi.org/10.1016/j.watres.2015.08.057
Qi W, Mei S, Yuan Y, Li X, Tang T, Zhao Q, Wu M, Wei W, Sun Y (2018) Enhancing fermentation wastewater treatment by co-culture of microalgae with volatile fatty acid- and alcohol-degrading bacteria. Algal Res 31:31–39. https://doi.org/10.1016/j.algal.2018.01.012
Higgins BT (2017) Algal-bacterial synergy in treatment of winery wastewater. NPJ Clean Water. https://doi.org/10.1038/s41545-018-0005-y
Ye J, Song Z, Wang L, Zhu J (2016) Metagenomic analysis of microbiota structure evolution in phytoremediation of a swine lagoon wastewater. Bioresour Technol 219:439–444. https://doi.org/10.1016/j.biortech.2016.08.013
Bernard O, Shoener BD, Schramm SM et al (2019) Microalgae and cyanobacteria modeling in water resource recovery facilities: a critical review. Water Res X 2:100024. https://doi.org/10.1016/j.wroa.2018.100024
Quijano G, Arcila JS, Buitrón G (2017) Microalgal-bacterial aggregates: applications and perspectives for wastewater treatment. Biotechnol Adv 35:772–781. https://doi.org/10.1016/j.biotechadv.2017.07.003
Zhai J, Li X, Li W, Rahaman MH, Zhao Y, Wei B, Wei H (2017) Optimization of biomass production and nutrients removal by Spirulina platensis from municipal wastewater. Ecol Eng 108:83–92. https://doi.org/10.1016/j.ecoleng.2017.07.023
Zhu S, Wu H, Wu C, Qiu G, Feng C, Wei C (2019) Structure and function of microbial community involved in a novel full-scale prefix oxic coking wastewater treatment O/H/O system. Water Res 164:114963. https://doi.org/10.1016/j.watres.2019.114963
Nagarajan D, Lee D, Chang J (2019) Integration of anaerobic digestion and microalgal cultivation for digestate bioremediation and biogas upgrading. Bioresour Technol 290:121804. https://doi.org/10.1016/j.biortech.2019.121804
Gram L (2018) A novel microbial culture chamber co-cultivation system to study algal-bacteria interactions using Emiliania huxleyi and Phaeobacter inhibens as model organisms. Front Microbiol 9. https://doi.org/10.3389/fmicb.2018.01705
Shirzad M, Karimi M, Silva JAC, Rodrigues E (2019) Moving bed reactors : challenges and progress of experimental and theoretical studies in a century of research. Ind Eng Chem Res 58:9179–9198. https://doi.org/10.1021/acs.iecr.9b01136
Orfanos AG, Manariotis ID (2019) Algal biofilm ponds for polishing secondary effluent and resource recovery. J Appl Phycol 31:1765–1772. https://doi.org/10.1007/s10811-018-1731-8
Keyvan A, Ahmadzadeh H, Farhad A et al (2016) Potential use of algae for heavy metal bioremediation, a critical review. J Environ Manag 181:817–831. https://doi.org/10.1016/j.jenvman.2016.06.059
Gentili FG, Fick J (2017) Algal cultivation in urban wastewater: an efficient way to reduce pharmaceutical pollutants. J Appl Phycol 29:255–262. https://doi.org/10.1007/s10811-016-0950-0
Sutherland DL, Ralph PJ (2019) Microalgal bioremediation of emerging contaminants-opportunities and challenges. Water Res 164:114921. https://doi.org/10.1016/j.watres.2019.114921
Asselborn V, Fernández C, Zalocar Y, Parodi ER (2015) Effects of chlorpyrifos on the growth and ultrastructure of green algae, Ankistrodesmus gracilis. Ecotoxicol Environ Saf 120:334–341. https://doi.org/10.1016/j.ecoenv.2015.06.015
Arbib Z, De Godos I, Ruiz J, Perales JA (2017) Optimization of pilot high rate algal ponds for simultaneous nutrient removal and lipids production. Sci Total Environ 589:66–72. https://doi.org/10.1016/j.scitotenv.2017.02.206
Boshir M, Zhou JL, Hao H et al (2016) Progress in the biological and chemical treatment technologies for emerging contaminant removal from wastewater: a critical review. J Hazard Mater 323:274–298. https://doi.org/10.1016/j.jhazmat.2016.04.045
Pal S, Banat F, Almansoori A, Haija MA (2016) Review of technologies for biotreatment of refinery wastewaters: progress, challenges and future opportunities. Environ Technol Rev 5:12–38. https://doi.org/10.1080/21622515.2016.1164252
Jassim SAA, Limoges RG (2016) Bacteriophage biocontrol in wastewater treatment. World J Microbiol Biotechnol 32:70. https://doi.org/10.1007/s11274-016-2028-1
Ratzke C, Barrere J, Gore J (2020) Strength of species interactions determines biodiversity and stability in microbial communities. Nat Ecol Evol 4:376–383. https://doi.org/10.1038/s41559-020-1099-4
Decho AW, Gutierrez T (2017) Microbial extracellular polymeric substances (EPSs) in ocean systems. Front Microbiol 8. https://doi.org/10.3389/fmicb.2017.00922
Marella TK, López-pacheco IY, Parra- R (2020) Wealth from waste: diatoms as tools for phycoremediation of wastewater and for obtaining value from the biomass. Sci Total Environ 724:137960. https://doi.org/10.1016/j.scitotenv.2020.137960
Liu J, Wu Y, Wu C, Muylaert K, Vyverman W, Yu HQ, Muñoz R, Rittmann B (2017) Advanced nutrient removal from surface water by a consortium of attached microalgae and bacteria: a review. Bioresour Technol 241:1127–1137. https://doi.org/10.1016/j.biortech.2017.06.054
Funding
This work was supported by the Basic Research Program for Natural Science of Shaanxi Province (2020JQ-714) “Study on the Growth-promoting Mechanism of Microalgae Symbiotic Bacteria on Chlorella in Wastewater Based on Quorum Sensing.”
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sial, A., Zhang, B., Zhang, A. et al. Microalgal–Bacterial Synergistic Interactions and Their Potential Influence in Wastewater Treatment: a Review. Bioenerg. Res. 14, 723–738 (2021). https://doi.org/10.1007/s12155-020-10213-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12155-020-10213-9