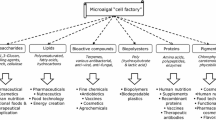
Abstract
Vegan diets preclude the availability of some of the essential fatty acids supplied by foods of animal origin. Significantly, eicosapentaenoic (EPA) and docosahexaenoic (DHA) acids are long-chain (LC)-omega − 3 (n − 3) polyunsaturated fatty acids (PUFAs), widely known for preventing a variety of metabolic diseases. In addition to vegan-food supplements, there is increasing demand for infant foods and health foods from dietary sources of EPA and DHA from plant origin. Their demands are being met industrially by utilizing thraustochytrids (marine protists) and microalgae-based platforms. The importance of these organisms is highlighted for the sustainable production of biotechnologically derived specialty lipids for human health.
Similar content being viewed by others
References
Saini RK, Keum YS (2018) Omega-3 and omega-6 polyunsaturated fatty acids: dietary sources, metabolism, and significance—a review. Life Sci 203:255–267. https://doi.org/10.1016/j.lfs.2018.04.049
Saini RK, Prasad P, Sreedhar RV, Akhilender Naidu K, Shang X, Keum Y-S (2021) Omega−3 polyunsaturated fatty acids (PUFAs): emerging plant and microbial sources, oxidative stability, bioavailability, and health benefits—a review. Antioxidants 10:1627. https://doi.org/10.3390/antiox10101627
EFSA Panel on Dietetic Products, Nutrition, and Allergies (NDA) (2010) Scientific opinion on dietary reference values for fats, including saturated fatty acids, polyunsaturated fatty acids, monounsaturated fatty acids, trans fatty acids, and cholesterol. EFSA J 8:1461. https://doi.org/10.2903/j.efsa.2010.1461
Del Gobbo LC, Imamura F, Aslibekyan S, Marklund M, Virtanen JK, Wennberg M, Yakoob MY, Chiuve SE, Dela Cruz L, Frazier-Wood AC et al (2016) ω-3 polyunsaturated fatty acid biomarkers and coronary heart disease: pooling project of 19 cohort studies. JAMA Intern Med 176:1155–1166. https://doi.org/10.1001/jamainternmed.2016.2925
Grosso G, Pajak A, Marventano S, Castellano S, Galvano F, Bucolo C, Drago F, Caraci F (2014) Role of omega-3 fatty acids in the treatment of depressive disorders: a comprehensive meta-analysis of randomized clinical trials. PLoS ONE 9:e96905. https://doi.org/10.1371/journal.pone.0096905
Swanson D, Block R, Mousa SA (2012) Omega-3 fatty acids EPA and DHA: health benefits throughout life. Adv Nutr 3:1–7. https://doi.org/10.3945/an.111.000893
Yan Y, Jiang W, Spinetti T, Tardivel A, Castillo R, Bourquin C, Guarda G, Tian Z, Tschopp J, Zhou R (2013) Omega-3 fatty acids prevent inflammation and metabolic disorder through inhibition of NLRP3 inflammasome activation. Immunity 38:1154–1163. https://doi.org/10.1016/j.immuni.2013.05.015
Simopoulos A (2016) An increase in the omega-6/omega-3 fatty acid ratio increases the risk for obesity. Nutrients 8:128. https://doi.org/10.3390/nu8030128
Yates CM, Calder PC, Ed Rainger G (2014) Pharmacology and therapeutics of omega-3 polyunsaturated fatty acids in chronic inflammatory disease. Pharmacol Ther 141:272–282. https://doi.org/10.1016/j.pharmthera.2013.10.010
Ryan AS, Astwood JD, Gautier S, Kuratko CN, Nelson EB, Salem N (2010) Effects of long-chain polyunsaturated fatty acid supplementation on neurodevelopment in childhood: a review of human studies. Prostaglandins. Leukotrienes Essential Fatty Acids (PLEFA) 82:305–314. https://doi.org/10.1016/j.plefa.2010.02.007
Xiang H, Sun-Waterhouse D, Waterhouse GIN, Cui C, Ruan Z (2019) Fermentation-enabled wellness foods: a fresh perspective. Food Sci Human Wellness 8:203–243. https://doi.org/10.1016/j.fshw.2019.08.003
Ryan L, Symington AM (2015) Algal-oil supplements are a viable alternative to fish-oil supplements in terms of docosahexaenoic acid (22:6n–3; DHA). J Funct Foods 19:852–858. https://doi.org/10.1016/j.jff.2014.06.023
Patel A, Karageorgou D, Katapodis P, Sharma A, Rova U, Christakopoulos P, Matsakas L (2021) Bioprospecting of thraustochytrids for omega-3 fatty acids: a sustainable approach to reduce dependency on animal sources. Trends Food Sci Tech 115:433–444. https://doi.org/10.1016/j.tifs.2021.06.044
Nethravathy MU, Mehar JG, Mudliar SN, Shekh AY (2019) Recent advances in microalgal bioactives for food, feed, and healthcare products: commercial potential, market space, and sustainability. Compr Rev Food Sci Food Saf 18:1882–1897. https://doi.org/10.1111/1541-4337.12500
Adarme-Vega T, Lim DKY, Timmins M, Vernen F, Li Y, Schenk PM (2012) Microalgal biofactories: a promising approach towards sustainable omega-3 fatty acid production. Microb Cell Fact 11:96. https://doi.org/10.1186/1475-2859-11-96
Ma X-N, Chen T-P, Yang B, Liu J, Chen F (2016) Lipid production from nannochloropsis. Mar Drugs 14:61. https://doi.org/10.3390/md14040061
Zanella L, Vianello F (2020) Microalgae of the genus nannochloropsis: chemical composition and functional implications for human nutrition. J Funct Foods 68:103919. https://doi.org/10.1016/j.jff.2020.103919
Paliwal C, Mitra M, Bhayani K, Bharadwaj SVV, Ghosh T, Dubey S, Mishra S (2017) Abiotic stresses as tools for metabolites in microalgae. Biores Technol 244:1216–1226. https://doi.org/10.1016/j.biortech.2017.05.058
Mitra M, Patidar SK, Mishra S (2015) Integrated process of two stage cultivation of Nannochloropsis sp. for nutraceutically valuable eicosapentaenoic acid along with biodiesel. Biores Technol 193:363–369. https://doi.org/10.1016/j.biortech.2015.06.033
Cui Y, Thomas-Hall SR, Chua ET, Schenk PM (2021) Development of high-level omega-3 eicosapentaenoic acid (EPA) production from phaeodactylum tricornutum. J Phycol 57:258–268. https://doi.org/10.1111/jpy.13082
Pocha CKR, Chia WY, Chew KW, Munawaroh HSH, Show PL (2022) Current advances in recovery and biorefinery of fucoxanthin from Phaeodactylum tricornutum. Algal Res 65:102735. https://doi.org/10.1016/j.algal.2022.102735
Gu W, Kavanagh JM, McClure DD (2022) Towards a sustainable supply of omega-3 fatty acids: screening microalgae for scalable production of eicosapentaenoic acid (EPA). Algal Res 61:102564. https://doi.org/10.1016/j.algal.2021.102564
Chi G, Xu Y, Ca, X, L, Z, Ca, M, Chist, Y, H, N (2022) Production of polyunsaturated fatty acids by Schizochytrium (Aurantiochytrium) spp. Biotechnology Advances 55, 107897, https://doi.org/10.1016/j.biotechadv.2021.107897.
Sun X-M, Xu Y-S, Huang H (2021) Thraustochytrid cell factories for producing lipid compounds. Trends Biotechnol 39:648–650. https://doi.org/10.1016/j.tibtech.2020.10.008
Huang TY, Lu WC, Chu IM (2012) A fermentation strategy for producing docosahexaenoic acid in Aurantiochytrium limacinum SR21 and increasing C22:6 proportions in total fatty acid. Biores Technol 123:8–14. https://doi.org/10.1016/j.biortech.2012.07.068
Gray RJ (2017) Application for the authorization of DHA and EPA-rich algal oil from Schizochytrium sp. Martek Biosciences Corporation, Columbia, Maryland, USA. https://acnfp.food.gov.uk/sites/default/files/mnt/drupal_data/sources/files/multimedia/pdfs/dhaoapplicdossier.pdf
Li J, Liu R, Chang G, Li X, ChangM LY, Jin Q, Wang X (2015) A strategy for the highly efficient production of docosahexaenoic acid by Aurantiochytrium limacinum SR21 using glucose and glycerol as the mixed carbon sources. Biores Technol 177:51–57. https://doi.org/10.1016/j.biortech.2014.11.046
Kiy T, Luy M, Zeumer O (2010) Production of omega-3 fatty acids in microflora of thraustochytriales using modified media. EP2084290B1.
Food Standards Australia New Zealand (FSANZ) (2003). DHASCO and ARASCO oils as sources of long-chain polyunsaturated fatty acids in infant formula; Food Standards Australia New Zealand: Canberra BC, Australia. https://www.foodstandards.govt.nz/publications/Documents/FSANZ_AR_05.pdf
Scott SD, Armenta RE, Berryman KT, Norman AW (2011) Use of raw glycerol to produce oil rich in polyunsaturated fatty acids by a thraustochytrid. Enzyme Microb Technol 48:267–272. https://doi.org/10.1016/j.enzmictec.2010.11.008
Gupta A, Barrow CJ, Puri M (2022) Multiproduct biorefinery from marine thraustochytrids towards a circular bioeconomy. Trends Biotechnol 40:448–462. https://doi.org/10.1016/j.tibtech.2021.09.003
Park H, Kwak M, Seo J, Ju J, Heo S, Park S, Hong W (2018) Enhanced production of carotenoids using a Thraustochytrid microalgal strain containing high levels of docosahexaenoic acid-rich oil. Bioprocess Biosyst Eng 41:1355–1370. https://doi.org/10.1007/s00449-018-1963-7
Yeiser M, Harris CL, Kirchoff AL, Patterson AC, Wampler JL, Zissman EN, Berseth CL (2016) Growth and tolerance of infants fed formula with a new algal source of docosahexaenoic acid: double-blind, randomized, controlled trial. Prostaglandins Leukot Essent Fatty Acids 115:89–96. https://doi.org/10.1016/j.plefa.2016.09.001
Hamilton HA, Newton R, Müller ANA, DB, (2020) Systems approach to quantify the global omega-3 fatty acid cycle. Nature Food 1:59–62. https://doi.org/10.1038/s43016-019-0006-0
Acknowledgements
This paper was supported by the KU research professor program of Konkuk University, Seoul, Republic of Korea.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Saini, R.K., Ravishankar, G.A. & Keum, Y. Microalgae and Thraustochytrids are Sustainable Sources of Vegan EPA and DHA with Commercial Feasibility. Indian J Microbiol 63, 155–158 (2023). https://doi.org/10.1007/s12088-023-01059-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-023-01059-8