Abstract
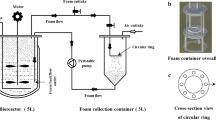
Rhamnolipid producing Pseudomonas sp. TMB2 was selected for this investigation to optimize the metabolite production in fabricated batch reactor after studying yield kinetics in shake-flask and tried to reduce the overall production cost through In-situ recovery technology. Using various kinetic models, maximum specific growth rate (μmax) and half velocity constant (KS) of TMB2 were determined to be 0.185 ± 0.0025 h−1 and 0.124 ± 0.024 g/L in shake-flask, respectively. Further, a batch reactor was designed with integration of a foam fractionate column in the lid of the vessel and their performances were compared with shake-flask studies. The yields of rhamnolipids production on biomass (YP/X), rhamnolipids production on substrate (YP/S) and biomass production on substrate (YX/S) were found to be higher in reactor than that of shake-flask. The best conditions for maximum rhamnolipid production in reactor were observed to be 2 vvm and 300 rpm, giving YP/S = 0.152 g/g, YP/X = 0.542 g/g and YX/S = 0.280 g/g. Rhamnolipid production was increased by ≈ 10.18% in the reactor than that of shake-flask in optimized conditions. Rhamnolipid concentrations in the foamate were also found to be higher than that of reactor vessels. Further, the performance of foam fractionation was validated through enrichment and recovery, which were found in the range of 2.75–4.86 and 25.33–64.64%, respectively.



Similar content being viewed by others
References
Haloi S, Medhi T (2020) Microbial enhanced oil recovery: a technological prospect. In: Gogoi SB (ed) Advances in petroleum technology. Jenny Stanford Publishing Pte. Ltd., Singapore, pp 157–182
Makkar RS, Cameotra SS, Banat IM (2011) Advances in utilization of renewable substrates for biosurfactant production. AMB Express 1:1–19. https://doi.org/10.1186/2191-0855-1-5
De S, Malik S, Ghosh A et al (2015) A review on natural surfactants. RSC adv 5:65757–65767. https://doi.org/10.1039/C5RA11101C
Tavares LF, Silva PM, Junqueira M et al (2013) Characterization of rhamnolipids produced by wild-type and engineered Burkholderia kururiensis. Appl Microbiol Biotechnol 97:1909–1921. https://doi.org/10.1007/s00253-012-4454-9
Haloi S, Medhi T (2019) Optimization and characterization of a glycolipid produced by Achromobacter sp. to use in petroleum industries. J Basic Microbiol 59:238–248. https://doi.org/10.1002/jobm.201800298
Ndlovu T, Rautenbach M, Vosloo JA et al (2017) Characterization and antimicrobial activity of biosurfactant extracts produced by Bacillus amyloliquefaciens and Pseudomonas aeruginosa isolated from a wastewater treatment plant. AMB Express 7:1–19. https://doi.org/10.1186/s13568-017-0363-8
Sood U, Singh DN, Hira P et al (2020) Rapid and solitary production of mono-rhamnolipid biosurfactant and biofilm inhibiting pyocyanin by a taxonomic outlier Pseudomonas aeruginosa strain CR1. J Biotechnol 307:98–106. https://doi.org/10.1016/j.jbiotec.2019.11.004
Haloi S, Sarmah S, Gogoi SB et al (2020) Characterization of Pseudomonas sp. TMB2 produced rhamnolipids for ex-situ microbial enhanced oil recovery. 3 Biotech 10:1–17. https://doi.org/10.1007/s13205-020-2094-9
Haloi S, Saikia MD, Gogoi SB et al (2021) Aggregation and static adsorption behaviour of Achromobacter sp. TMB1 produced rhamnolipids on sandstone core in relation to Microbial enhanced oil recovery. J Pet Sci Eng 205:108831. https://doi.org/10.1016/j.petrol.2021.108831
Datta P, Tiwari P, Pandey LM (2018) Isolation and characterization of biosurfactant producing and oil degrading Bacillus subtilis MG495086 from formation water of Assam oil reservoir and its suitability for enhanced oil recovery. Bioresour Technol 270:439–448. https://doi.org/10.1016/j.biortech.2018.09.047
Singh V, Saha S, Padmanabhan P (2020) Assessment of the wettability of hydrophobic solid substrate by biosurfactant produced by Bacillus aryabhattai SPS1001. Curr Microbiol 77:1716–1723. https://doi.org/10.1007/s00284-020-01985-6
Pathania AS, Jana AK (2020) Improvement in production of rhamnolipids using fried oil with hydrophilic co-substrate by Indigenous Pseudomonas aeruginosa NJ2 and Characterizations. Appl Biochem Biotechnol 191:1223–1246. https://doi.org/10.1007/s12010-019-03221-9
Partovi M, Lotfabad TB, Roostaazad R et al (2013) Management of soybean oil refinery wastes through recycling them for producing biosurfactant using Pseudomonas aeruginosa MR01. World J Microbiol Biotechnol 29:1039–1047. https://doi.org/10.1007/s11274-013-1267-7
Chen C, Sun N, Li D et al (2018) Optimization and characterization of biosurfactant production from kitchen waste oil using Pseudomonas aeruginosa. Environ Sci Pollut Res 25:14934–14943. https://doi.org/10.1007/s11356-018-1691-1
Md Noh NA, Mohd Salleh S, Yahya ARM (2014) Enhanced rhamnolipid production by Pseudomonas aeruginosa USM-AR 2 via fed-batch cultivation based on maximum substrate uptake rate. Lett Appl Microbiol 58:617–623. https://doi.org/10.1111/lam.12236
Betts JPJ, Warr SRC, Finka GB et al (2014) Impact of aeration strategies on fed-batch cell culture kinetics in a single-use 24-well miniature bioreactor. Biochem Eng J 82:105–116. https://doi.org/10.1016/j.bej.2013.11.010
Yao S, Zhao S, Lu Z et al (2015) Control of agitation and aeration rates in the production of surfactin in foam overflowing fed-batch culture with industrial fermentation. Rev Argent Microbiol 47:344–349. https://doi.org/10.1016/j.ram.2015.09.003
Beuker J, Steier A, Wittgens A et al (2016) Integrated foam fractionation for heterologous rhamnolipid production with recombinant Pseudomonas putida in a bioreactor. AMB Express 6:1–10. https://doi.org/10.1186/s13568-016-0183-2
Shuler ML (1992) Bioprocess enzineering; In Prentice-Hall, 412–420
Nicolai SP (1999) Kinetics, microbial growth. In: Encyclopedia of bioprocess technology: fermentation, biocatalysis and bioseparation. pp 1513–1543
Marchant R, Banat IM (2014) Protocols for measuring biosurfactant production in microbial cultures. In: McGenity T, Timmis K, Nogales B (eds) Hydrocarbon and lipid microbiology protocols. Springer, Heidelberg. https://doi.org/10.1007/8623_2014_10
Winterburn JB, Martin PJ (2012) Foam mitigation and exploitation in biosurfactant production. Biotechnol Lett 34:187–195. https://doi.org/10.1007/s10529-011-0782-6
Campos JM, Montenegro Stamford TL, Sarubbo LA et al (2013) Microbial biosurfactants as additives for food industries. Biotechnol prog 29:1097–1108. https://doi.org/10.1002/btpr.1796
Lin SC, Carswell KS, Sharma MM et al (1994) Continuous production of the lipopeptide biosurfactant of Bacillus licheniformis JF-2. Appl Microbiol Biotechnol 41:281–285. https://doi.org/10.1007/BF00221219
Sarachat T, Pornsunthorntawee O, Chavadej S et al (2010) Purification and concentration of a rhamnolipid biosurfactant produced by Pseudomonas aeruginosa SP4 using foam fractionation. Bioresour technol 101:324–330. https://doi.org/10.1016/j.biortech.2009.08.012
Amani H, Mehrnia MR, Sarrafzadeh MH et al (2010) Scale up and application of biosurfactant from Bacillus subtilis in enhanced oil recovery. Appl Biochem Biotechnol 162:510–523. https://doi.org/10.1007/s12010-009-8889-0
Acknowledgements
The authors are grateful to Prof. N. N. Dutta, Guest faculty, Department of Molecular Biology and Biotechnology, Tezpur University for technical advice during preparation of the manuscript.The author Saurav Haloi humbly acknowledges Late Dr. Tapas Medhi, Ph.D. supervisor, for his invaluable guidance, support and understanding and pray for the departed soul to attain eternal peace.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Haloi, S., Medhi, T. Kinetics and Production of Rhamnolipid from Pseudomonas sp. TMB2 in Shake-Flask and Fabricated Batch Reactor. Indian J Microbiol 62, 434–440 (2022). https://doi.org/10.1007/s12088-022-01021-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-022-01021-0