Abstract
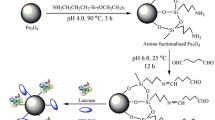
In the present study, Rhus vernicifera laccase (RvLac) was immobilized through covalent methods on the magnetic nanoparticles. Fe2O3 and Fe3O4 nanoparticles activated by 3-aminopropyltriethoxysilane followed with glutaraldehyde showed maximum immobilization yields and relative activity up to 81.4 and 84.3% at optimum incubation and pH of 18 h and 5.8, respectively. The maximum RvLac loading of 156 mg/g of support was recorded on Fe2O3 nanoparticles. A higher optimum pH and temperature of 4.0 and 45 °C were noted for immobilized enzyme compared to values of 3.5 and 40 °C for free form, respectively. Immobilized RvLac exhibited better relative activity profiles at various pH and temperature ranges. The immobilized enzyme showed up to 16-fold improvement in the thermal stability, when incubated at 60 °C, and retained up to 82.9% of residual activity after ten cycles of reuses. Immobilized RvLac exhibited up to 1.9-fold higher bisphenol A degradation efficiency potential over free enzyme. Previous reports have demonstrated the immobilization of RvLac on non-magnetic supports. This study has demonstrated that immobilization of RvLac on magnetic nanoparticles is very efficient especially for achieving high loading, better pH and temperature profiles, and thermal- and solvents-stability, high reusability, and higher degradation of bisphenol A.







Similar content being viewed by others
References
Shukla P (2019) Synthetic biology perspectives of microbial enzymes and their innovative applications. Indian J Microbiol 59:401–409. https://doi.org/10.1007/s12088-019-00819-9
Singh DN, Sood U, Singh AK, Gupta V, Shakarad M, Rawat CD, Lal R (2019) Genome sequencing revealed the biotechnological potential of an obligate thermophile Geobacillus thermoleovorans strain RL isolated from hot water spring. Indian J Microbiol 59:351–355. https://doi.org/10.1007/s12088-019-00809-x
Anwar MZ, Kim DJ, Kumar A, Patel SKS, Otari S, Mardina P, Jeong JH, Sohn JH, Kim JH, Park JT, Lee JK (2017) SnO2 hollow nanotubes: a novel and efficient support matrix for enzyme immobilization. Sci Rep 7:15333. https://doi.org/10.1038/s41598-017-15550-y
Lee J-K, Patel SKS, Sung BH, Kalia VC (2020) Biomolecules from municipal and food industry wastes: an overview. Bioresour Technol 298:122346. https://doi.org/10.1016/j.biortech.2029.122346
Zhang C, You S, Liu Y, Wang C, Yan Q, Qi W, Su R, He Z (2020) Construction of luffa sponge-based magnetic carbon nanocarriers for laccase immobilization and its application in the removal of bisphenol A. Bioresour Technol 305:123085. https://doi.org/10.1016/j.biortech.2020.123085
Gao H, Li J, Sivakumar D, Kim T-S, Patel SKS, Kalia VC, Kim I-W, Zhang Y-W, Lee J-K (2019) NADH oxidase from Lactobacillus reuteri: a versatile enzyme for oxidized cofactor regeneration. Int J Biol Macromol 123:629–636. https://doi.org/10.1016/j.ijbiomac.2018.11.096
Kondaveeti S, Patel SKS, Woo J, Wee JH, Kim S-Y, Al-Raoush RI, Kim I-W, Kalia VC, Lee J-K (2019) Characterization of cellobiohydrolases from Schizophyllum commune KMJ820. Indian J Microbiol 60:160–166. https://doi.org/10.1007/s12088-019-00843-9
Kumar V, Patel SKS, Gupta RK, Otari SV, Gao H, Lee JK, Zhang L (2019) Enhanced saccharification and fermentation of rice straw by reducing the concentration of phenolic compounds using an immobilized enzyme cocktail. Biotechnol J 14:1800468. https://doi.org/10.1002/biot.201800468
Panday D, Patel SKS, Singh R, Kumar P, Thakur V, Chand D (2019) Solvent-tolerant acyltransferase from Bacillus sp. APB-6: purification and characterization. Indian J Microbiol 59:500–507. https://doi.org/10.1007/s12088-019-00836-8
Fernández-Fernández M, Sanromán MÁ, Moldes D (2013) Recent developments and applications of immobilized laccase. Biotechnol Adv 31:1808–1825. https://doi.org/10.1016/j.biotechadv.2012.02.013
Kim T-S, Patel SKS, Selvaraj C, Jung W-S, Pan C-H, Kang YC, Lee J-K (2016) A highly efficient sorbitol dehydrogenase from Gluconobacter oxydans G624 and improvement of its stability through immobilization. Sci Rep 6:33438. https://doi.org/10.1038/srep33438
Patel SKS, Kumar V, Mardina P, Li J, Lestari R, Kalia VC, Lee J-K (2018) Methanol production from simulated biogas mixtures by co-immobilized Methylomonas methanica and Methylocella tundrae. Bioresour Technol 263:25–32. https://doi.org/10.1016/j.biortech.2018.04.096
Patel SKS, Kalia VC, Joo JB, Kang YC, Lee J-K (2020) Biotransformation of methane into methanol by methanotrophs immobilized on coconut coir. Bioresour Technol 297:122433. https://doi.org/10.1016/j.biortech.2019.122433
Patel SKS, Shanmugam R, Kalia VC, Lee J-K (2020) Methanol production by polymer-encapsulated methanotrophs from simulated biogas in the presence of methane vector. Bioresour Technol 304:123022. https://doi.org/10.1016/j.biortech.2020.123022
Otari SV, Patel SKS, Kim S-Y, Haw JR, Kalia VC, Kim I-W, Lee J-K (2019) Copper ferrite magnetic nanoparticles for the immobilization of enzyme. Indian J Microbiol 59:105–108. https://doi.org/10.1007/s12088-018-0768-3
Durate D, Casadio R, Martelli L, Tasco G, Portaccio M, Luca PD, Bencivenga U, Rossi S, Martino SD, Grano V, Diano N, Mita DG (2004) Isothermal and non-isothermal bioreactors in the detoxification of waste waters polluted by aromatic compounds by means of immobilised laccase from Rhus vernicifera. J Mol Catal B Enzym 27:191–206. https://doi.org/10.1016/j.molcatb.2003.11.008
Patel SKS, Choi SH, Kang YC, Lee J-K (2017) Eco-friendly composite of Fe3O4-reduced graphene oxide particles for efficient enzyme immobilization. ACS Appl Mater Inter 9:2213–2222. https://doi.org/10.1021/acsami.6b05165
Kumar A, Kim I-W, Patel SKS, Lee J-K (2018) Synthesis of protein-inorganic nanohybrids with improved catalytic properties using Co3(PO4)2. Indian J Microbiol 58:100–104. https://doi.org/10.1007/s12088-017-0700-2
Kumar A, Patel SKS, Mardan B, Pagolu R, Lestari R, Jeong S-H, Kim T, Haw JR, Lim S-Y, Kim I-W, Lee J-K (2018) Immobilization of xylanase using a protein-inorganic hybrid system. J Microbiol Biotechnol 28:638–644. https://doi.org/10.4014/jmb.1710.10037
Patel SKS, Gupta RK, Kumar V, Mardina P, Lestari R, Kalia VC, Choi M-S, Lee J-K (2019) Influence of metal ions on the immobilization of β-glucosidase through protein-inorganic hybrids. Indian J Microbiol 59:370–374. https://doi.org/10.1007/s12088-019-0796-z
Patel SKS, Choi SH, Kang YC, Lee J-K (2016) Large-scale aerosol-assisted synthesis of biofriendly Fe2O3 yolk-shell particles: a promising support for enzyme immobilization. Nanoscale 8:6728–6738. https://doi.org/10.1039/C6NR00346J
Patel SKS, Anwar MZ, Kumar A, Otari SV, Pagolu RT, Kim S-Y, Kim I-W, Lee J-K (2018) Fe2O3 yolk-shel particle-based laccase biosensor for efficient detection of 2,6-dimethoxyphenol. Biochem Eng J 132:1–8. https://doi.org/10.1016/j.bej.2017.12.013
Kumar A, Park GD, Patel SKS, Kondaveeti S, Otari S, Anwar MZ, Kalia VC, Singh Y, Kim SC, Cho B-K, Sohn J-H, Kim DR, Kang YC, Lee J-K (2019) SiO2 microparticles with carbon nanotube-derived mesopores as an efficient support for enzyme immobilization. Chem Eng J 359:1252–1264. https://doi.org/10.1016/j.cej.2018.11.052
Patel SKS, Kalia VC, Choi JH, Haw JR, Kim IW, Lee JK (2014) Immobilization of laccase on SiO2 nanocarriers improves its stability and reusability. J Microbiol Biotechnol 24:639–647. https://doi.org/10.4014/jmb.1401.01025
Patel SKS, Otari SV, Kang YC, Lee JK (2017) Protein-inorganic hybrid system for efficient his-tagged enzymes immobilization and its application in L-xylulose production. RSC Adv 7:3488–3494. https://doi.org/10.1039/c6ra24404a
Yang WY, Min DY, Wen SX, Jin L, Rong L, Tetsuo M, Chen Bo (2006) Immobilization and characterization of laccase from Chinese Rhus vernicifera on modified chitosan. Process Biochem 41:1378–1382. https://doi.org/10.1016/j.procbio.2006.01.018
Otari SV, Patel SKS, Kalia VC, Lee J-K (2020) One-step hydrothermal synthesis of magnetic rice straw for effective lipase immobilization and its application in esterification reaction. Bioresour Technol 302:122887. https://doi.org/10.1016/j.biortech.2020.122887
Lu R, Miyakoshi T (2012) Studies on acetone powder and purified Rhus laccase immobilized on zirconium chloride for oxidation of phenols. Enzyme Res 2012:375309. https://doi.org/10.1155/2012/375309
Ran F, Zou Y, Xu Y, Liu X, Zhang H (2019) Fe3O4@MoS2@PEI-facilitated enzyme tethering for efficient removal of persistent organic pollutants in water. Chem Eng J 375:121947. https://doi.org/10.1016/j.cej.2019.121947
Suman SK, Patnam PL, Ghosh S, Jain SL (2019) Chicken feather derived novel support material for immobilization of laccase and its application in oxidation of veratryl alcohol. ACS Sustain Chem Eng 7:3464–3474. https://doi.org/10.1021/acssuschemeng.8b05679
Kondaveeti S, Pagolu R, Patel SKS, Kumar A, Bisht A, Dad D, Kalia VC, Kim I-W, Lee J-K (2019) Bioelectrochemical detoxification of phenolic compounds during enzymatic pre-treatment of rice straw. J Microbiol Biotechnol 29:1760–1768. https://doi.org/10.4014/jmb.1909.09042
Sharma KK, Kuhad RC (2008) Laccase: enzyme revisited and function redefined. Indian J Microbiol 48:309. https://doi.org/10.1007/s12088-008-0028-z
Gupta V, Capalash N, Gupta N, Sharma P (2017) Bio-prospecting laccases in the bacterial diversity of activated sludge from pulp and paper industry. Indian J Microbiol 57:75–82. https://doi.org/10.1007/s12088-016-0624-2
Singh G, Bhalla A, Kaur P, Capalash N, Sharma P (2011) Laccase from prokaryotes: a new source for an old enzyme. Rev Environ Sci Biotechnol 10:309–326. https://doi.org/10.1007/s11157-011-9257-4
Singh G, Kaur K, Puri S, Sharma P (2015) Critical factors affecting laccase-mediated biobleaching of pulp in paper industry. Appl Microbiol Biotechnol 99:155–164. https://doi.org/10.1007/s00253-014-6219-0
Georgieva S, Godjevargova T, Portaccio M, Lepore M, Mita DG (2008) Advantages in using non-isothermal bioreactors in bioremediation of water polluted by phenol by means of immobilized laccase from Rhus vernicifera. J Mol Catal B Enzym 55:177–184. https://doi.org/10.1016/j.molcatb.2008.03.011
Olshansky Y, Masaphy S, Root RA, Rytwo G (2018) Immobilization of Rhus vernicifera laccase on sepiolite; effect of chitosan and copper modification on laccase adsorption and activity. Appl Clay Sci 152:143–147. https://doi.org/10.1016/j.clay.2017.11.006
Mogharabi-Manzari M, Heydari M, Sadeghian-Abadi S, Yousefi-Mokri M, Faramarzi MA (2019) Enzymatic dimerization of phenylacetylene by laccase immobilized on magnetic nanoparticles via click chemistry. Biocatal Biotransform 37:455–465. https://doi.org/10.1080/10242422.2019.1611788
Wan Y-Y, Lu R, Akiyama K, Okamoto K, Honda T, Du Y-M, Yoshida T, Miyakoshi T, Knill CJ, Kennedy JF (2010) Effects of lacquer polysaccharides, glycoproteins and isoenzymes on the activity of free and immobilised laccase from Rhus vernicifera. Int J Biol Macromol 47:76–81. https://doi.org/10.1016/j.ijbiomac.2010.03.016
Patel SKS, Gupta RK, Kondaveeti S, Otari SV, Kumar A, Kalia VC, Lee J-K (2020) Conversion of biogas to methanol by methanotrophs immobilized on chemically modified chitosan. Bioresour Technol 315:123791. https://doi.org/10.1016/j.biortech.2020.123791
Kondaveeti S, Patel SKS, Pagolu R, Li J, Kalia VC, Choi M-S, Lee J-K (2019) Conversion of simulated biogas to electricity: sequential operation of methanotrophic reactor effluents in microbial fuel cell. Energy 189:116309. https://doi.org/10.1016/j.energy.2019.116309
Patel SKS, Choi H, Lee J-K (2019) Multimetal-based inorganic–protein hybrid system for enzyme immobilization. ACS Sustain Chem Eng 7:13633–13638. https://doi.org/10.1021/acssuschemeng.9b02583
Patel SKS, Kim J-H, Kalia VC, Lee J-K (2019) Antimicrobial activity of amino-derivatized cationic polysaccharides. Indian J Microbiol 59:96–99. https://doi.org/10.1007/s12088-018-00764-7
Patel SKS, Ray S, Prakash J, Wee JH, Kim S-Y, Lee J-K, Kalia VC (2019) Co-digestion of biowastes to enhance biological hydrogen process by defined mixed bacterial cultures. Indian J Microbiol 59:154–160. https://doi.org/10.1007/s12088-018-00777-8
Patel SKS, Jeon MS, Gupta RK, Jeon Y, Kalia VC, Kim SC, Cho B-K, Kim DR, Lee J-K (2019) Hierarchical macro-porous particles for efficient whole-cell immobilization: application in bioconversion of greenhouse gases to methanol. ACS Appl Mater Interfaces 11:18968–18977. https://doi.org/10.1021/acsami.9b03420
Rouhani S, Rostami A, Salimi A, Pourshiani O (2018) Graphene oxide/CuFe2O4 nanocomposite as a novel scaffold for the immobilization of laccase and its application as a recyclable nanobiocatalyst for the green synthesis of arylsulfonyl benzenediols. Biochem Eng J 133:1–11. https://doi.org/10.1016/j.bej.2018.01.004
Tarasi R, Alipour M, Gorgannezhad L, Imanparast S, Yousefi-Ahmadipour A, Ramezani A, Ganjali MR, Shafiee A, Faramarzi MA, Khoobi M (2018) Laccase immobilization onto magnetic β-cyclodextrin-modified chitosan: improved enzyme stability and efficient performance for phenolic compounds elimination. Macromol Res 26:755–762. https://doi.org/10.1007/s13233-018-6095-z
Patel SKS, Otari SV, Li J, Kim DR, Kim SC, Cho B-K, Kalia VC, Kang YC, Lee J-K (2018) Synthesis of cross-linked protein-metal hybrid nanoflowers and its application in repeated batch decolorization of synthetic dyes. J Hazard Mater 347:442–450. https://doi.org/10.1016/j.jhazmat.2018.01.003
Singh G, Bhalla A, Capalash N, Sharma P (2010) Characterization of immobilized laccase from γ-proteobacterium JB: approach towards the development of biosensor for the detection of phenolic compounds. Indian J Sci Technol 3:48–53. https://doi.org/10.17485/ijst/2010/v3i1/29643
Angural S, Rana M, Sharma A, Warmoota R, Puri N, Gupta N (2020) Combinatorial biobleaching of mixedwood pulp with lignolytic and hemicellulolytic enzymes for paper making. Indian J Microbiol 60:383–387. https://doi.org/10.1007/s12088-020-00867-6
Acknowledgements
This research was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Science, ICT & Future Planning (2019R1F1A1063131, 2020H1D3A2A01060467, 2017R1A2B3011676). This work was also supported by KU Research Professor Program of Konkuk University.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Patel, S.K.S., Gupta, R.K., Kim, SY. et al. Rhus vernicifera Laccase Immobilization on Magnetic Nanoparticles to Improve Stability and Its Potential Application in Bisphenol A Degradation. Indian J Microbiol 61, 45–54 (2021). https://doi.org/10.1007/s12088-020-00912-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12088-020-00912-4