Abstract
Background
Immune related cells are known to be closely related to the therapeutic effects and prognoses of cancer patients. In this study, we analyzed immune cell profiles (ICP) of cholangiocarcinoma patients (CCA).
Methods
To measure the frequency of immune cells, peripheral blood mononuclear cells of 41 CCA and 10 healthy volunteers (HV) were analyzed by FACS.
Results
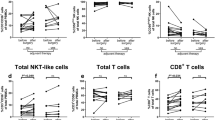
There were significant differences between CCA and HV in ICP, and these differences were a consequence of tumor-bearing status, because many items in ICP before surgery were restored to levels in HV after surgery. Therefore, these changes were specifically attributable to cholangiocarcinoma, and we examined if they can function as biomarkers for therapeutic effects and prognoses. A shorter overall survival was associated with a lower frequency of helper T cells (HT) (p = 0.001), a higher frequency of effector regulatory T cells (eTregs) (p = 0.008), and a lower frequency of CD80 + eTregs (p = 0.024) in the best supportive care group, with a lower frequency of CD25 + naïve Tregs (nTregs) (p = 0.005) in the chemotherapy group, and with a lower frequency of OX40 + HT (p = 0.022), CD25 + CD8 + T cells (p = 0.017), and OX40 + CD8 + T cells (p = 0.032) in the surgery group. The recurrence factors were a higher frequency of CD4 + T cells (p = 0.009), CCR6 + nTregs (p = 0.014), and CXCR3 + nTregs (p = 0.012), and a lower frequency of PD-1 + HT (p = 0.006), OX40 + HT (p = 0.004), CD8 + T cells (p = 0.001), and CTLA-4 + CD8 + T cells (p = 0.036).
Conclusions
The ICP in CCA are specifically attributable to cholangiocarcinoma, and may be biomarkers for therapeutic effects and prognoses.
Graphical abstract





Similar content being viewed by others
Data availability
There are no linked research data sets for this submission. The following reason is given: Date will be available on request.
Abbreviations
- CCA:
-
Cholangiocarcinoma patients
- CTLs:
-
Cytotoxic T lymphocytes
- HCC:
-
Hepatocellular carcinoma
- PBMCs:
-
Peripheral blood mononuclear cells
- Tregs:
-
Regulatory T cells
- MDSCs:
-
Myeloid-derived suppressor cells
- ICP:
-
Immune cell profiles
- CD:
-
Anti-cluster of differentiation
- HV:
-
Healthy volunteers
- HCV SVR12:
-
Hepatitis C virus sustained virological response 12
- iCCA:
-
Intrahepatic cholangiocarcinoma
- pCCA:
-
Perihilar cholangiocarcinoma
- GBCA:
-
Gallbladder carcinoma including cystic duct carcinoma
- dCCA:
-
Distal cholangiocarcinoma
- BSC:
-
Best supportive care
- HBV:
-
Hepatitis B virus infection
- HCV:
-
HCV infection
- DM:
-
Diabetes mellitus
- UICC:
-
Union Internationale Contre Le Cancer
- HLA:
-
Human histocompatibility leukocyte antigen
- FoxP3:
-
Forkhead box P3
- CTLA-4:
-
Cytotoxic T-lymphocyte antigen-4
- PD-1:
-
Programmed cell death-1
- PD-L1:
-
Programmed cell death-1 ligand
- CCR:
-
C–C chemokine receptor
- CXCR3:
-
C-X-C motif chemokine receptor 3
- AST:
-
Aspartate transaminase
- ALT:
-
Alanine transaminase
- γGTP:
-
γ-Glutamyltranspeptidase
- WBC:
-
White blood cell
- ALP:
-
Alkaline phosphatase
- T.bil:
-
Total bilirubin
- CA19-9:
-
Carbohydrate antigen 19-9
- nTregs:
-
Naïve Tregs
- eTregs:
-
Effector Tregs
- OS:
-
Overall survival
- CEA:
-
Carcinoembryonic antigen
- RFS:
-
Recurrence-free survival
- FSC:
-
Forward scatter
- SSC:
-
Side scatter
- pre:
-
Pre-operation group
- post:
-
Post-operation group
- NR:
-
Non-recurrent cholangiocarcinoma patient group
- R:
-
Recurrent cholangiocarcinoma patient group
- before:
-
Before biliary drainage group
- after:
-
After biliary drainage group
- Freq:
-
Frequency
- M:
-
Male
- F:
-
Female
- D.bil:
-
Direct bilirubin
- CRP:
-
C-reactive protein
- PSC:
-
Primary sclerosing cholangitis
- ND:
-
Not determined
- FCS:
-
Heat-inactivated fetal calf serum
References
Khan SA, Davidson BR, Goldin RD, et al. Guidelines for the diagnosis and treatment of cholangiocarcinoma. Gut 2012;61:1657–1669
Sawada Y, Yoshikawa T, Nobuoka D, et al. Phase I trial of a glypican-3-derived peptide vaccine for advanced hepatocellular carcinoma: immunologic evidence and potential for improving overall survival. Clin Cancer Res 2012;18:3686–3696
Kida A, Mizukoshi E, Tamai T, et al. Immune responses against tumour-associated antigen-derived cytotoxic T lymphocyte epitopes in cholangiocarcinoma patients. Liver Int 2018;38:2040–2050
Inada Y, Mizukoshi E, Seike T, et al. Characteristics of immune response to tumor-associated antigens and immune cell profile in patients with hepatocellular carcinoma. Hepatology 2019;69:653–665
Saito T, Nishikawa H, Wada H, et al. Two FOXP3+CD4+ T cell subpopulations distinctly control the prognosis of colorectal cancers. Nat Med 2016;22:679–684
Murakami Y, Uemura K, Sudo T, et al. Prognostic factors after surgical resection for intrahepatic, hilar, and distal cholangiocarcinoma. Ann Surg Oncol 2011;18:651–658
Marshall NB, Swain SL. Cytotoxic CD4 T cells in antiviral immunity. J Biomed Biotechnol 2011;2011:954602
Borst J, Ahrends T, Bąbała N, et al. CD4+ T cell help in cancer immunology and immunotherapy. Nat Rev Immunol 2018;18:635–647
Liyanage UK, Moore TT, Joo HG, et al. Prevalence of regulatory T cells is increased in peripheral blood and tumor microenvironment of patients with pancreas or breast adenocarcinoma. J Immunol 2002;169:2756–2761
Woo EY, Chu CS, Goletz TJ, et al. Regulatory CD4(+)CD25(+) T cells in tumors from patients with early-stage non-small cell lung cancer and late-stage ovarian cancer. Cancer Res 2001;61:4766–4772
Vignali DA, Collison LW, Workman CJ. How regulatory T cells work. Nat Rev Immunol 2008;8:523–532
Whiteside TL. The role of regulatory T cells in cancer immunology. Immunotargets Ther 2015;4:159–171
Woo EY, Yeh H, Chu CS, et al. Cutting edge: Regulatory T cells from lung cancer patients directly inhibit autologous T cell proliferation. J Immunol 2002;168:4272–4276
Voo KS, Foglietta M, Percivalle E, et al. Selective targeting of Toll-like receptors and OX40 inhibit regulatory T-cell function in follicular lymphoma. Int J Cancer 2014;135:2834–2846
Ohue Y, Nishikawa H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci 2019;110:2080–2089
Ihara F, Sakurai D, Horinaka A, et al. CD45RA-Foxp3high regulatory T cells have a negative impact on the clinical outcome of head and neck squamous cell carcinoma. Cancer Immunol Immunother 2017;66:1275–1285
Rech AJ, Mick R, Martin S, et al. CD25 blockade depletes and selectively reprograms regulatory T cells in concert with immunotherapy in cancer patients. Sci Transl Med 2012;4:134
Chaput N, Louafi S, Bardier A, et al. Identification of CD8+CD25+Foxp3+ suppressive T cells in colorectal cancer tissue. Gut 2009;58:520–529
Ito T, Wang YH, Duramad O, et al. OX40 ligand shuts down IL-10-producing regulatory T cells. Proc Natl Acad Sci USA 2006;103:13138–13143
Lee JJ, Kao KC, Chiu YL, et al. Enrichment of human CCR6+ regulatory T cells with superior suppressive activity in oral cancer. J Immunol 2017;199:467–476
Bromley SK, Mempel TR, Luster AD. Orchestrating the orchestrators: chemokines in control of T cell traffic. Nat Immunol 2008;9:970–980
Galon J, Costes A, Sanchez-Cabo F, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 2006;313:1960–1964
Acknowledgements
The authors thank Taro Kawane, Aya Ito, Michiko Nishino, Saiho Sugimoto, Toshiki Matsuo, Atsuyoshi Mizukami, Masaaki Yano, Fumitaka Arihara, Hironori Hayashi, Koji Amaya, Koichiro Matsuda, Kohei Ogawa, and Mitsuru Matsuda from the Department of Internal Medicine and Surgery of Toyama Prefectural Central Hospital for collecting cholangiocarcinoma samples.
Funding
This study was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan (25460984).
Author information
Authors and Affiliations
Contributions
Conception and design: AK, EM, TT, KA, TY, TY, YS, MH, and SK. Development of methodology: EM, and SK. Acquisition of data: AK, AU, AS, and KS. Analysis and interpretation of date: AK, and TT. Writing and review of the manuscript: AK, and EM. Administrative, technical, or material support: AK, HK and KF. Study supervision: SK.
Corresponding author
Ethics declarations
Conflict of interest
Akihiko Kida declared that no conflict of interest exists. Eishiro Mizukoshi declared that no conflict of interest exists. Hidenori Kido declared that no conflict of interest exists. Tadashi Toyama declared that no conflict of interest exists. Takeshi Terashima declared that no conflict of interest exists. Kuniaki Arai declared that no conflict of interest exists. Tatsuya Yamashita declared that no conflict of interest exists. Kazumi Fushimi declared that no conflict of interest exists. Taro Yamashita declared that no conflict of interest exists. Yoshio Sakai declared that no conflict of interest exists. Masao Honda declared that no conflict of interest exists. Akio Uchiyama declared that no conflict of interest exists. Akito Sakai declared that no conflict of interest exists. Koichi Shimizu declared that no conflict of interest exists. Shuichi Kaneko declared that no conflict of interest exists.
Ethics approval
This study was approved by the ethics committee of Kanazawa University (No.1237), and all patients provided written informed consent to participate in accordance with the Declaration of Helsinki.
Consent to participate
All patients provided written informed consent to participate in this study.
Consent for publication
Consent for publication was received from the patients.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kida, A., Mizukoshi, E., Kido, H. et al. The characteristics of the immune cell profiles in peripheral blood in cholangiocarcinoma patients. Hepatol Int 15, 695–706 (2021). https://doi.org/10.1007/s12072-021-10177-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12072-021-10177-8