Abstract
Objective
The subset distribution and immunophenotype of circulating immune cells (“peripheral blood immune cell profile”) may reflect tumor development and response to cancer treatment. In order to use the peripheral blood immune cell profile as biomarker to monitor patients over time, it is crucial to know how immune cell subsets respond to therapeutic interventions. In this study, we investigated the effects of tumor resection and adjuvant therapy on the peripheral blood immune cell profile in patients with colon carcinoma (CC).
Methods
The subset distribution and immunophenotype of T cells (CD3+CD56−), CD56dim NK cells (CD3−CD56dim), CD56bright NK cells (CD3−CD56bright) and NKT-like cells (CD3+CD56+) were studied in preoperative and postoperative peripheral blood mononuclear cell (PBMC) samples of 24 patients with CC by multiparameter flow cytometry. Changes in immunophenotype of circulating immune cells after tumor resection were studied in patients treated with and without (capecitabine-based) adjuvant therapy.
Results
The NKT-like cell (% of total PBMCs) and CD8+ T cell (% of total T cells) populations expanded in the peripheral blood of non-adjuvant-treated CC patients after surgery. NK- and NKT-like cells showed upregulation of activating receptors and downregulation of inhibitory receptors in non-adjuvant-treated CC patients after surgery. These changes were not observed in the peripheral blood of adjuvant-treated CC patients.
Conclusions
Our results suggest tumor-induced suppression of NK- and NKT-like cells in CC patients, an effect that could not be detected after tumor resection. In contrast, adjuvant therapy maintained tumor-induced immunosuppression of NK- and NKT-like cells in CC patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Colon carcinoma (CC) is a tumor type with a high incidence and, combined with rectal cancer, globally accounting for more than 1 million new cases and around 600.000 deaths annually [1,2,3]. Despite improved surgical procedures and adjuvant treatment strategies, around 30% of the patients, without metastatic disease at time of diagnosis, develop recurrence or dissemination of the disease following successful resection of the primary tumor [4]. Currently, different biomarkers are evaluated for their prognostic value that may be used to identify cancer patients at risk of disease progression. Hence, biomarker application in cancer patients is crucial since it may provide information for therapeutic decision making, including decisions on the frequency of clinical follow-up, thereby possibly prolonging survival and reducing the risk of recurrence in cancer patients. Over the past decades, it has become clear that immunosurveillance plays a pivotal role in colon cancer by protecting the host against tumor development and progression. For instance, the immunoscore, representing CD3+/CD8+ T lymphocyte density in tumor tissue, is a strong prognostic factor in colorectal cancer (CRC) [5,6,7,8,9]. Low density of cytotoxic (CD8+) tumor-infiltrating T cells was reported to correlate with poor clinical outcome in CRC [5, 6, 10, 11]. However, it must be realized that the immunoscore can only be determined once at time of surgery because it is performed on resection material. Therefore, it does not provide information regarding treatment response of patients over time. In general, immune cells are present in relatively low numbers in the tissue of primary tumors, whereas they are abundantly present in peripheral blood. Interestingly, the subset distribution and immunophenotype of circulating immune cells (“peripheral blood immune cell profile”) are associated with clinical outcome of CRC patients [12]. In contrast to the immunoscore, the peripheral blood immune cell profile can be monitored in patients over time since it only requires blood sampling.
The peripheral blood comprises different immune cell subsets, among which are natural killer (NK) cells that can be subdivided based on their CD56 expression. CD56dim NK cells have an important cytotoxic function, while CD56bright NK cells are generally associated with production of pro-inflammatory cytokines and immunoregulatory properties [13, 14]. Furthermore, peripheral blood contains a unique natural killer T (NKT) cell population with characteristics of both NK cells and NKT cells. Co-expression of CD3 and CD56 can be used to identify this immune subset in the circulation using flow cytometry, which is often referred to as “NKT-like” [15]. Like other immune cell subsets, NK- and NKT-like cell activity is dependent on a delicate balance between activating and inhibitory signals from cell surface receptors [16]. The activating signals are mediated by a wide array of receptors including natural killer group 2-C (NKG2C), natural killer group 2-D (NKG2D), DNAX accessory molecule-1 (DNAM-1), CD161, and natural cytotoxicity receptors NKp30, NKp44, and NKp46 that recognize a variety of stress-induced molecules that may be present on tumor cells. Additionally, the CD16 (FcγRIII) receptor mediates antibody-dependent cell-mediated cytotoxicity and CD8 enhances the cytolytic activity of NK cells [17, 18]. NK cell inhibitory receptors include natural killer group 2-A (NKG2A) and killer cell immunoglobulin (Ig)-like receptors CD158a and CD158b that recognize human leukocyte antigen (HLA) class I molecules. Recently, we [12] and others [19,20,21] have shown that the peripheral blood immune cell profile is altered in CRC patients compared to healthy donors, characterized by downregulation of activating receptors on circulating CD56dim NK cells and NKT-like cells.
Several studies reported a critical role for the tumor microenvironment (TME) in shaping NK- and NKT-like receptor-mediated anti-tumor immunity [15, 22, 23]. For instance, immune-modulating effects of NK cells have been attributed to hypoxic conditions [24] and immunosuppressive cytokines and signaling molecules [23, 25, 26] present in the TME. As a result of these immune modulations, NK- and NKT-like cells may downregulate activating receptors and, as a result, may become functionally impaired, thereby promoting tumor escape. Up till now, it is unclear whether TME-induced immune-modulations of circulating NK- and NKT-like cells are reversible. Furthermore, chemotherapy, often used for adjuvant treatment of CRC patients, has been reported to have an immunosuppressive effect on the immune system, including overall impairment of NK cell responses [27]. In order to use the peripheral blood immune cell profile as biomarker to monitor cancer patients over time, it is crucial to know how immune cell subsets respond to therapeutic interventions. In a recent paper [12], we studied the preoperative peripheral blood immune cell profile in patients with CRC. In this study, we investigated the peripheral blood immune cell profile in patients with available postoperative blood samples in order to study the effects of tumor resection and adjuvant therapy. It was not possible to study the effects of tumor resection and adjuvant therapy in patients with rectum tumors due to limited sample availability. Therefore, we focused on patients with colon tumors. Additionally, we selected patients with Tumor–node–metastasis (TNM) stage II and III who are at risk for development of metastases after surgery and are, therefore, the patients that would qualify for biomarker monitoring over time. In summary, our collection of peripheral blood mononuclear cells (PBMCs) before and after tumor resection enabled, for the first time, to study the effects of tumor resection as well as adjuvant therapy on the subset distribution and immunophenotype of circulating T-, NK- and NKT-like cells in CC patients.
Materials and methods
Study population
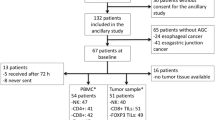
The study population comprised patients diagnosed with CC who underwent surgical resection of their primary tumor at the Leiden University Medical Center (LUMC, the Netherlands) between 2001 and 2007. Patients were included in this study based on availability of PBMC samples. PBMC samples were collected within a month prior to surgery and ≥ 2 months after surgery (mean 8.2 months after surgery, range 2.2–17.7). When the patient started adjuvant therapy directly after surgery, postoperative PBMC samples were obtained after therapy completion. These samples were obtained ≥ 5 months after the final therapy date (mean 8.4 months after surgery, range 6.7–13.7). Most adjuvant therapy cycles consisted of 3 weeks of therapy; therefore, the included postoperative PBMC samples were obtained ≥ 4 months after therapy completion. In the present study, patients with histologically proven primary CC, TNM stage II and III, surgical R0 resection, and a minimal amount of 5 million cryopreserved preoperative as well as postoperative PBMCs were included (N = 26). Clinicopathological data of all patients were available. All blood samples were obtained after approval by the Medical Ethical Committee of the LUMC (protocol number P000.193). All procedures performed in this study were in accordance with the ethical standards of the Dutch law (“WMO”, medical research involving human subjects act), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. All CC patients included in this study agreed to our use of their PBMCs and data for research purposes prior to blood sampling via written informed consent, and they moreover agreed to anonymous publication of the resulting data.
Isolation of peripheral blood mononuclear cells
PBMCs from CC patients were isolated and cryopreserved as previously described [12]. PBMCs were also isolated from the buffy coat of a random blood donor obtained from the Blood Bank at Aarhus University Hospital (Dept. Clinical Immunology, Aarhus University Hospital, Skejby, Denmark), which was used as an internal control in the flow cytometry experiments [12].
Flow cytometry antibody staining and data analysis
All preoperative PBMC samples from CC patients were previously immunophenotyped and reported on as part of a CRC study [12]. All postoperative PBMC samples selected for the current study were immunophenotyped following the same protocol. Briefly, PBMC samples were thawed following a standard protocol and the cell concentration was adjusted to 10 × 106 cells/mL. PBMCs were then blocked for 15–30 min at room temperature with 50 μg/mL human IgG (CSL Behring, Bern, Switzerland) and subsequently stained with two distinct antibody panels containing selected NK- and NKT cell markers as previously described [12]. After the staining procedure, samples were fixed in PBS supplemented with 0.9% formaldehyde (Sigma-Aldrich, St. Louis, MO) and analyzed the same day by flow cytometry on a 4-laser equipped LSRFortessa (BD Biosciences, San Diego, CA) using Diva 7.0 software (BD Biosciences). Results were analyzed with FlowJo software v10.1 (Tree Star Inc., Ashland, OR). The buffy coat was used as an internal control in order to check for any inter-experimental variation. Expression of phenotypic markers on peripheral blood T-, NK- and NKT-like cells was evaluated by the median fluorescence intensity (MFI) and/or the percentage of positive cells. A compensation matrix was calculated to compensate for spillover of signal into other channels in the multicolor flow cytometry experiments. This matrix was set up using single stains on CompBeads (BD Biosciences,) OneComp eBeads (eBioscience, San Diego, CA), CompBeads Plus (BD Biosciences) and ArC reactive beads (Life Technologies). A standardized gating strategy based on a healthy donor buffy coat was used in order to study the distribution of markers on specific lymphocyte subsets as described previously [12].
Statistical analyses
Statistical analyses were conducted using SPSS statistical software (IBM SPSS Statistics 23, Chicago, USA). The age of patients treated with adjuvant therapy was compared with the age of patients treated without adjuvant therapy using the Mann–Whitney U test. Preoperative and postoperative PBMC samples from patients were compared using the paired samples T test and Wilcoxon signed-rank test, for normally distributed and not normally distributed variables, respectively. We corrected for multiple testing using the Benjamini–Hochberg method, by which adjusted P-values were calculated (indicated by P*) [28]. P*-values ≤ 0.05 were considered statistically significant.
Results
Study population
The subset distribution and immunophenotype of circulating T-, NK- and NKT-like cells were studied pre- and postoperatively in patients diagnosed with CC TNM stage II and III (N = 26). One postoperative sample was excluded due to low viability of the PBMCs (< 50% viable cells). Additionally, one patient was diagnosed with Lynch syndrome. Since tumors from patients with Lynch syndrome are immunologically different compared to tumors from patients with sporadic cancer [29], this patient was excluded from analyses, resulting in a total study population of 24 patients. One patient had undergone local radiotherapy prior to the collection of the preoperative PBMC sample. In total, 15 patients underwent surgical resection of their tumor only (non-adjuvant-treated group) and 9 patients received additional therapy after surgery (adjuvant-treated group). Two of these 9 patients were treated with capecitabine monotherapy, six patients were treated with a combination of capecitabine and oxaliplatin, and one patient was treated with a combination of capecitabine, oxaliplatin and bevacizumab. Table 1 summarizes the clinicopathological characteristics of the 24 patients included in the analyses. Patients undergoing adjuvant therapy were younger (median 56 years) compared to patients that did not receive adjuvant therapy (median 73 years, P = 0.002). The low number of patients did not allow for analyzing the peripheral blood immune cell profile according to age.
Expansion of NKT-like and CD8+ T cell populations in peripheral blood after surgery in non-adjuvant-treated CC patients
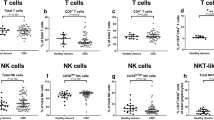
First, the effects of tumor resection and adjuvant therapy on the distribution of peripheral blood immune subsets were studied in non-adjuvant-treated (N = 15) and adjuvant-treated (N = 9) CC patients (Table 2). The percentages of total NK cells (% CD3−CD56+ cells of total PBMCs), CD56dim NK cells (% of total NK cells), and CD56bright NK cells (% of total NK cells) did neither change in non-adjuvant-treated patients nor in adjuvant-treated patients after resection of the primary tumor (Fig. 1a–c, respectively). The percentage of NKT-like cells (% CD3+CD56+ cells of total PBMCs) increased (P* = 0.049) in non-adjuvant-treated patients after surgery (Fig. 1d). Furthermore, although the percentage of total T cells (% CD3+CD56− cells of total PBMCs) was not altered in non-adjuvant-treated patients after resection of the primary tumor (Fig. 1e), the percentage of CD8+ T cells (% of total T cells) was significantly increased (P* = 0.034, Fig. 1f). No change was observed in the percentage of NKT-like cells or CD8+ T cells in adjuvant-treated patients after surgery.
Effects of tumor resection and adjuvant therapy on the peripheral blood immune cell subset distribution in CC patients. Multiparameter flow cytometry was used to study the peripheral blood immune profile in PBMC samples from CC patients. The distribution of circulating immune cell subsets was compared before and after surgery in CC patients that did (N = 9) or did not (N = 15) receive adjuvant therapy. a Total percentage of NK cells (% CD3−CD56+ cells of total PBMCs). b Percentage of CD56dim NK cells (% of total NK cells). c Percentage of CD56bright NK cells (% of total NK cells). d Total percentage of NKT-like cells (% CD3+CD56+ cells of total PBMCs). e Total percentage of T cells (% CD3+CD56− cells of total PBMCs). f Percentage of CD8+ T cells (% of total T cells). Pre- and postoperative samples from each patient are connected with a line. P*-values ≤ 0.05 were considered statistically significant. CC Colon carcinoma, PBMCs peripheral blood mononuclear cells, NK natural killer, NKT natural killer T, ns not significant
Increased expression of activating receptors and downregulation of inhibitory receptors on NK cells in peripheral blood after surgery in non-adjuvant-treated CC patients
After analyzing the distribution of immune cell subsets, we compared the immunophenotype of NK- and NKT-like cells before and after tumor resection in non-adjuvant-treated and adjuvant-treated CC patients (Table 2). Within the CD56dim NK cell population, the expression levels of activating receptors NKp44 (P* = 0.036) and NKG2D (P* = 0.045) increased after surgery in non-adjuvant-treated patients (Fig. 2a, b, respectively). Additionally, a trend was observed towards a higher expression level of the co-stimulatory molecule CD8 in non-adjuvant-treated patients after surgery (P* = 0.060) (Fig. 2c). The expression levels of NKp44, NKG2D and CD8 did not change on CD56dim NK cells after tumor resection in adjuvant-treated patients (Fig. 2a–c, respectively). In line with these results, a trend towards increased expression levels of NKG2D (P* = 0.051) and CD8 (P* = 0.051) was observed on CD56bright NK cells in non-adjuvant-treated patients (Fig. 3a, b, respectively). No change in expression levels of NKG2D and CD8 was observed on CD56bright NK cells after tumor resection in adjuvant-treated patients. The expression level of the inhibitory receptor NKG2A (P* = 0.045) on CD56bright cells decreased in non-adjuvant-treated patients after surgery (Fig. 3c). In contrast, the percentage of stimulatory receptor CD16+ CD56bright NK cells was also decreased (P* = 0.040) in non-adjuvant-treated patients after tumor resection (Fig. 3d). The expression level of NKG2A and the percentage of CD16+ cells did not change after surgery in adjuvant-treated patients. In summary, circulating NK cells acquired expression of cell surface markers associated with functional activity after surgery in non-adjuvant-treated CC patients as compared to before surgery.
Effects of tumor resection and adjuvant therapy on the immunophenotype of CD56dim NK cells in CC patients. Multiparameter flow cytometry was used to study the peripheral blood immune profile in PBMC samples from CC patients. The immunophenotype of CD56dim NK cells was compared before and after surgery in CC patients that did (N = 9) or did not (N = 15) receive adjuvant therapy. a Expression level of NKp44 on CD56dim NK cells. b Expression level of NKG2D on NKG2D+CD56dim NK cells. c Expression level of CD8 on CD8+CD56dim NK cells. Pre- and postoperative samples from each patient are connected with a line. Statistically significant P*-values are indicated in bold. CC Colon carcinoma, MFI median fluorescence intensity, NK natural killer, ns not significant
Effects of tumor resection and adjuvant therapy on the immunophenotype of CD56bright NK cells in CC patients. Multiparameter flow cytometry was used to study the peripheral blood immune profile in PBMC samples from CC patients. The immunophenotype of CD56bright NK cells was compared before and after surgery in CC patients that did (N = 9) or did not (N = 15) receive adjuvant therapy. a Expression level of NKG2D on NKG2D+CD56bright NK cells. b Expression level of CD8 on CD8+CD56bright cells. c Expression level of NKG2A on NKG2A+CD56bright NK cells. d Percentage of CD16+CD56bright NK cells. Pre- and postoperative samples from each patient are connected with a line. Statistically significant P*-values are indicated in bold. CC Colon carcinoma, MFI median fluorescence intensity, NK natural killer, ns not significant
Increased expression of activating receptors and downregulation of inhibitory receptors on NKT-like cells in peripheral blood after surgery in non-adjuvant-treated CC patients
Within the NKT-like cell subset, the percentages of NKT-like cells expressing the inhibitory receptors CD158a or NKG2A decreased in non-adjuvant-treated patients after surgery (P* = 0.048 and P* = 0.030, respectively), whereas no change was observed in adjuvant-treated patients (Fig. 4a, b). In contrast, the expression levels of activating receptors NKG2D (P* = 0.048) and co-stimulatory receptor CD8 (P* = 0.030) on NKT-like cells increased in non-adjuvant-treated patients after surgery (Fig. 4c, d). No change in NKT-like cell expression levels of NKG2D and CD8 was observed after surgery in adjuvant-treated patients (Fig. 4c, d). Additionally, an increased percentage of NKp44+NKT-like cells was observed in non-adjuvant-treated patients after surgery (P* = 0.040, Fig. 4e). The expression level of NKp44 on NKT-like cells showed a similar pattern as the percentage of NKp44+NKT-like cells and was increased in non-adjuvant-treated patients after surgery (P* = 0.038, Fig. 4f). The percentage of NKp44+NKT-like cells, as well as the expression level of NKp44 on NKT-like cells, did not change in adjuvant-treated patients after surgery (Fig. 4e, f). In summary, as also observed for the immunophenotype of NK cells, NKT-like cells acquired expression of cell surface markers associated with functional activity after surgery in non-adjuvant-treated CC patients.
Effects of tumor resection and adjuvant therapy on the immunophenotype of NKT-like cells in CC patients. Multiparameter flow cytometry was used to study the peripheral blood immune profile in PBMC samples from CC patients. The immunophenotype of NKT-like cells was compared before and after surgery in CC patients that did (N = 9) or did not (N = 15) receive adjuvant therapy. a Percentage of CD158a+NKT-like cells. b Percentage of NKG2A+NKT-like cells. c Expression level of NKG2D on NKG2D+NKT-like cells. d Expression level of CD8 on CD8+NKT-like cells. e Percentage of NKp44+NKT-like cells. f Expression level of NKp44 on NKT-like cells. Pre- and postoperative samples from each patient are connected with a line. Statistically significant P*-values are indicated in bold. CC Colon carcinoma, CI confidence interval, MFI median fluorescence intensity, NKT natural killer T, ns not significant
Discussion
As shown in our recent study [12], the peripheral blood immune cell profile comprises a potential pool of biomarkers in CRC. We hypothesized that this profile might change upon therapeutic interventions. Therefore, we studied the peripheral blood immune profile in available postoperative PBMC samples (N = 24) from TNM stage II and III CC patients included in our previous study [12]. Our collection of PBMCs before and after tumor resection enabled, for the first time, to study the effects of tumor resection as well as adjuvant therapy on the subset distribution and immunophenotype of circulating immune cells in CC. Here, we focused on NK- and NKT-like cell subsets as several studies have reported an altered phenotype of these cells in peripheral blood of CRC patients compared to healthy donors, characterized by downregulation of activating receptors, which suggests impaired function of these immune cell subsets in cancer patients [12, 19, 20]. A critical role of the TME was implied in shaping NK- and NKT-like receptor-mediated anti-tumor immunity [15, 22, 23], which could be a result of hypoxic conditions, immunosuppressive cytokines or fibroblasts present in the TME [23,24,25,26].
We observed changes in peripheral blood immune cell profile after resection of the primary tumor in non-adjuvant-treated patients, including expansion of NKT-like (% of total PBMCs) and CD8+ T (% of total T cells) cell populations. An increase in percentage of these cell types was also observed after surgery in non-adjuvant-treated patients with laryngeal cancer [30]. Importantly, the reported expansion of the NKT-like and CD8+ T cell populations in the present study and that by Klatka et al. [30] is relative and does not provide information about a change in absolute numbers of these immune subsets. It was not possible to assess cells/ml counts in the present study since our samples were frozen PBMCs, not whole blood. Thus, trying to make measurements of cells/ml would be highly affected by the Ficoll procedure and sample preparation. This is a limitation of our study and should be taken into account when drawing conclusions. Grimm et al. [31] reported no absolute increase in CD8+ T cells in patients with oral squamous cell carcinoma (OSCC) after surgery, suggesting no effect on absolute immune cell numbers after surgery. However, it must be realized that this study was focused on OSCC patients that might have a different preoperative peripheral blood immune profile compared to CC patients which might also respond different to tumor resection. Furthermore, OSCC patients are often treated with neoadjuvant radiotherapy which might influence the immune system over a long period of time. The study by Grimm et al. did not specify neoadjuvant and/or adjuvant treatment of the included OSCC patients in their study. Up till now, studies reported on the numbers of circulating immune cells and subset distribution in cancer patients after surgery [30,31,32], but not on the immunophenotype of specific immune cell subsets. It is important that this is taken into account since the immunophenotype of immune cells is closely related to their function. Hence, expansion of the NKT-like cell population in CC patients after surgery does not necessarily mean that more effector cells are present. In this study, we showed that expression of activating receptors including NKG2D, NKp44 and CD8 was upregulated on NKT-like cells after surgery in non-adjuvant-treated patients. Additionally, we observed a decrease in the percentage of inhibitory receptor CD158a+ and NKG2A+ NKT-like cells, suggesting that the NKT-like cell population expands and acquires expression of cell surface markers associated with functional activity in CC patients after tumor resection. In contrast to studies on laryngeal cancer [30] and oral squamous cell carcinoma [31], we did not observe expansion of the NK cell population after tumor resection. We did, however, observe a change in immunophenotype of NK cells resembling the pattern observed in NKT-like cells. Hence, we observed upregulation of activating receptors NKG2D, NKp44 and CD8 on CD56dim NK cells in non-adjuvant-treated CC patients after tumor resection. In line with this, upregulation of activating receptors NKG2D and CD8 was observed on CD56bright NK cells as well as downregulation of the inhibitory receptor NKG2A after tumor resection in non-adjuvant-treated CC patients, suggesting that both CD56dim NK and CD56bright NK cells acquired a more active phenotype after tumor resection and, therefore, possibly recovered from TME-induced immunosuppression. The only activating marker that was downregulated in CC patients after tumor resection was the percentage of CD16+ cells within the CD56bright NK cell population, suggesting differences in TME-induced recovery of CD56dim and CD56bright NK cells. This is in line with a previous study in which we also reported differences in immunophenotype of CD56dim in comparison with CD56bright NK cells in preoperative CRC patients [12], implicating different roles for NK cell subsets in patients, thereby emphasizing the need to analyze these subsets separately.
Several studies investigated short-term effects of surgical resection on the immune system in cancer patients (1–14 days after surgery) [32,33,34,35,36], whereas only a few studies focused on the long-term effects (≥ 6 weeks after surgery). Although these studies did not investigate the expression of different activating and inhibitory cell surface receptors, they all report expansion of cytotoxic immune cell subsets after surgery, including NK cells, NKT cells and CD8+ T cells [30,31,32], suggesting a more active immune system in patients after tumor resection, which is in line with our results. Importantly, the changes in immunophenotype of NK- and NKT-like cells observed in our study after surgery were restricted to patients that did not receive any adjuvant therapy, suggesting that adjuvant therapy delays or even prevents the recovery of TME-induced suppression of NK- and NKT-like cells. As the postoperative samples from adjuvant-treated patients were obtained ≥ 4 months after therapy completion, this implies long lasting immunosuppressive effects of adjuvant therapy. This is in line with a study in breast cancer patients that reported decreased numbers of CD4+ T cells and B cells even 9 months after completion of chemotherapy [37].
The fact that therapeutic interventions influenced the peripheral blood immune cell profile in CC patients has consequences regarding its biomarker potential. In an ideal situation, biomarkers such as circulating immune cells could be used to monitor treatment response and risk of recurrence in patients over time. Our data suggest that TME-induced immunosuppression is de-activated after resection of the primary tumor. In theory, patients without complete resection of the tumor or patients with micro-metastases after tumor resection still have a TME present which could mean that TME-induced immunosuppression of NK- and NKT-like cells is not removed. Despite the small sample size of our cohort and statistical correction for multiple testing, we still found significant results in our study. Further research is required to investigate whether the peripheral blood immune cell profile can be used as biomarker to determine the presence of residual tumor cells in cancer patients.
In conclusion, we observed expansion of NKT-like cells and CD8+ T cells and activation of both NK- and NKT-like cells after tumor resection in CC patients. This suggests TME-induced suppression of NK cells and NKT-like cells, an effect that could not be detected after surgical resection of the primary tumor. The changes in peripheral blood immune cell profile after tumor resection were restricted to patients that did not receive any adjuvant therapy, suggesting that adjuvant therapy delays or even prevents the recovery of TME-induced suppressed NK- and NKT-like cells in CC patients after surgery. These observations are of importance for using the peripheral blood immune cell profile as a biomarker in CC, but should also be considered when developing cancer immunotherapy for CC patients.
Abbreviations
- CC:
-
Colon carcinoma
- CRC:
-
Colorectal cancer
- DNAM-1:
-
DNAX accessory molecule-1
- HLA:
-
Human leukocyte antigen
- LUMC:
-
Leiden University Medical Center
- MFI:
-
Median fluorescence intensity
- NK:
-
Natural killer
- NKG2A:
-
Natural killer group 2-A
- NKG2C:
-
Natural killer group 2-C
- NKG2D:
-
Natural killer group 2-D
- NKT:
-
Natural killer T
- OSCC:
-
Oral squamous cell carcinoma
- PBMC:
-
Peripheral blood mononuclear cell
- TME:
-
Tumor microenvironment
- TNM:
-
Tumor–node–metastasis
References
Ferlay J, Parkin DM, Steliarova-Foucher E (2010) Estimates of cancer incidence and mortality in Europe in 2008. Eur J Cancer 46(4):765–781. https://doi.org/10.1016/j.ejca.2009.12.014
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J, Rosso S, Coebergh JW, Comber H, Forman D, Bray F (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49(6):1374–1403. https://doi.org/10.1016/j.ejca.2012.12.027
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D, Bray F (2015) Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136(5):E359–386. https://doi.org/10.1002/ijc.29210
van Cutsem E, Cervantes A, Nordlinger B, Arnold D, ESMO Guidelines Working Group (2014) Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol Suppl 3:1–9
Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F (2006) Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science 313(5795):1960–1964. https://doi.org/10.1126/science.1129139
Mlecnik B, Tosolini M, Kirilovsky A, Berger A, Bindea G, Meatchi T, Bruneval P, Trajanoski Z, Fridman WH, Pages F, Galon J (2011) Histopathologic-based prognostic factors of colorectal cancers are associated with the state of the local immune reaction. J Clin Oncol 29(6):610–618. https://doi.org/10.1200/JCO.2010.30.5425
Finn OJ (2012) Host response in tumor diagnosis and prognosis: importance of immunologists and pathologists alliance. Exp Mol Pathol 93(3):315–318. https://doi.org/10.1016/j.yexmp.2012.10.013
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Nagtegaal ID, Palmqvist R, Masucci GV, Botti G, Tatangelo F, Delrio P, Maio M, Laghi L, Grizzi F, Asslaber M, D'Arrigo C, Vidal-Vanaclocha F, Zavadova E, Chouchane L, Ohashi PS, Hafezi-Bakhtiari S, Wouters BG, Roehrl M, Nguyen L, Kawakami Y, Hazama S, Okuno K, Ogino S, Gibbs P, Waring P, Sato N, Torigoe T, Itoh K, Patel PS, Shukla SN, Wang Y, Kopetz S, Sinicrope FA, Scripcariu V, Ascierto PA, Marincola FM, Fox BA, Pages F (2014) Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 232(2):199–209. https://doi.org/10.1002/path.4287
Mlecnik B, Bindea G, Angell HK, Maby P, Angelova M, Tougeron D, Church SE, Lafontaine L, Fischer M, Fredriksen T, Sasso M, Bilocq AM, Kirilovsky A, Obenauf AC, Hamieh M, Berger A, Bruneval P, Tuech JJ, Sabourin JC, Le Pessot F, Mauillon J, Rafii A, Laurent-Puig P, Speicher MR, Trajanoski Z, Michel P, Sesboue R, Frebourg T, Pages F, Valge-Archer V, Latouche JB, Galon J (2016) Integrative analyses of colorectal cancer show Immunoscore is a stronger predictor of patient survival than microsatellite instability. Immunity 44(3):698–711. https://doi.org/10.1016/j.immuni.2016.02.025
Galon J, Mlecnik B, Bindea G, Angell HK, Berger A, Lagorce C, Lugli A, Zlobec I, Hartmann A, Bifulco C, Pagès F (2014) Towards the introduction of the 'Immunoscore' in the classification of malignant tumours. J Pathol 232(2):199–209
Pages F, Mlecnik B, Marliot F, Bindea G, Ou FS, Bifulco C, Lugli A, Zlobec I, Rau TT, Berger MD, Nagtegaal ID, Vink-Borger E, Hartmann A, Geppert C, Kolwelter J, Merkel S, Grutzmann R, Van den Eynde M, Jouret-Mourin A, Kartheuser A, Leonard D, Remue C, Wang JY, Bavi P, Roehrl MHA, Ohashi PS, Nguyen LT, Han S, MacGregor HL, Hafezi-Bakhtiari S, Wouters BG, Masucci GV, Andersson EK, Zavadova E, Vocka M, Spacek J, Petruzelka L, Konopasek B, Dundr P, Skalova H, Nemejcova K, Botti G, Tatangelo F, Delrio P, Ciliberto G, Maio M, Laghi L, Grizzi F, Fredriksen T, Buttard B, Angelova M, Vasaturo A, Maby P, Church SE, Angell HK, Lafontaine L, Bruni D, El Sissy C, Haicheur N, Kirilovsky A, Berger A, Lagorce C, Meyers JP, Paustian C, Feng Z, Ballesteros-Merino C, Dijkstra J, van de Water C, van Lent-van Vliet S, Knijn N, Musina AM, Scripcariu DV, Popivanova B, Xu M, Fujita T, Hazama S, Suzuki N, Nagano H, Okuno K, Torigoe T, Sato N, Furuhata T, Takemasa I, Itoh K, Patel PS, Vora HH, Shah B, Patel JB, Rajvik KN, Pandya SJ, Shukla SN, Wang Y, Zhang G, Kawakami Y, Marincola FM, Ascierto PA, Sargent DJ, Fox BA, Galon J (2018) International validation of the consensus Immunoscore for the classification of colon cancer: a prognostic and accuracy study. Lancet 391 (10135):2128–2139. https://doi.org/10.1016/S0140-6736(18)30789-X
Krijgsman D, de Vries NL, Skovbo A, Andersen MN, Swets M, Bastiaannet E, Vahrmeijer AL, van de Velde CJH, Heemskerk MHM, Hokland M, Kuppen PJK (2019) Characterization of circulating T-, NK-, and NKT cell subsets in patients with colorectal cancer: the peripheral blood immune cell profile. Cancer Immunol Immunother. https://doi.org/10.1007/s00262-019-02343-7
Cooper MA, Fehniger TA, Caligiuri MA (2001) The biology of human natural killer-cell subsets. Trends Immunol 22(11):633–640
Jonges LE, Albertsson P, van Vlierberghe RL, Ensink NG, Johansson BR, van de Velde CJ, Fleuren GJ, Nannmark U, Kuppen PJ (2001) The phenotypic heterogeneity of human natural killer cells: presence of at least 48 different subsets in the peripheral blood. Scand J Immunol 53(2):103–110
Krijgsman D, Hokland M, Kuppen PJK (2018) The role of natural killer T cells in cancer-a phenotypical and functional approach. Front Immunol 9:367. https://doi.org/10.3389/fimmu.2018.00367
Biassoni R, Cantoni C, Pende D, Sivori S, Parolini S, Vitale M et al (2001) Human natural killer cell receptors and co-receptors. Immunol Rev 181:203–214
Dutertre CA, Bonnin-Gelize E, Pulford K, Bourel D, Fridman WH, Teillaud JL (2008) A novel subset of NK cells expressing high levels of inhibitory FcgammaRIIB modulating antibody-dependent function. J Leukoc Biol 84(6):1511–1520. https://doi.org/10.1189/jlb.0608343
Addison EG, North J, Bakhsh I, Marden C, Haq S, Al-Sarraj S, Malayeri R, Wickremasinghe RG, Davies JK, Lowdell MW (2005) Ligation of CD8alpha on human natural killer cells prevents activation-induced apoptosis and enhances cytolytic activity. Immunology 116(3):354–361. https://doi.org/10.1111/j.1365-2567.2005.02235.x
Peng YP, Zhu Y, Zhang JJ, Xu ZK, Qian ZY, Dai CC et al (2013) Comprehensive analysis of the percentage of surface receptors and cytotoxic granules positive natural killer cells in patients with pancreatic cancer, gastric cancer, and colorectal cancer. J Transl Med 11:262. https://doi.org/10.1186/1479-5876-11-262
Rocca YS, Roberti MP, Arriaga JM, Amat M, Bruno L, Pampena MB et al (2013) Altered phenotype in peripheral blood and tumor-associated NK cells from colorectal cancer patients. Innate Immun 19(1):76–85. https://doi.org/10.1177/1753425912453187
Gharagozloo M, Rezaei A, Kalantari H, Bahador A, Hassannejad N, Maracy M, Nouri N, Sedghi M, Ghazanfari H, Bayat B (2018) Decline in peripheral blood NKG2D+CD3+CD56+ NKT cells in metastatic colorectal cancer patients. Bratisl Lek Listy 119(1):6–11. https://doi.org/10.4149/BLL_2018_002
Baginska J, Viry E, Paggetti J, Medves S, Berchem G, Moussay E, Janji B (2013) The critical role of the tumor microenvironment in shaping natural killer cell-mediated anti-tumor immunity. Front Immunol 4:490. https://doi.org/10.3389/fimmu.2013.00490
Vitale M, Cantoni C, Pietra G, Mingari MC, Moretta L (2014) Effect of tumor cells and tumor microenvironment on NK-cell function. Eur J Immunol 44(6):1582–1592. https://doi.org/10.1002/eji.201344272
Balsamo M, Manzini C, Pietra G, Raggi F, Blengio F, Mingari MC, Varesio L, Moretta L, Bosco MC, Vitale M (2013) Hypoxia downregulates the expression of activating receptors involved in NK-cell-mediated target cell killing without affecting ADCC. Eur J Immunol 43(10):2756–2764. https://doi.org/10.1002/eji.201343448
Han B, Mao FY, Zhao YL, Lv YP, Teng YS, Duan M, Chen W, Cheng P, Wang TT, Liang ZY, Zhang JY, Liu YG, Guo G, Zou QM, Zhuang Y, Peng LS (2018) Altered NKp30, NKp46, NKG2D, and DNAM-1 Expression on Circulating NK Cells Is Associated with Tumor Progression in Human Gastric Cancer. J Immunol Res 2018:6248590. https://doi.org/10.1155/2018/6248590
Valayer A, Brea D, Lajoie L, Avezard L, Combes-Soia L, Labas V, Korkmaz B, Thibault G, Baranek T, Si-Tahar M (2017) Neutrophils can disarm NK cell response through cleavage of NKp46. J Leukoc Biol 101(1):253–259. https://doi.org/10.1189/jlb.3AB0316-140RR
Bracci L, Schiavoni G, Sistigu A, Belardelli F (2014) Immune-based mechanisms of cytotoxic chemotherapy: implications for the design of novel and rationale-based combined treatments against cancer. Cell Death Differ 21(1):15–25. https://doi.org/10.1038/cdd.2013.67
Benjamini Y, Hochberg Y (1995) Controlling the false discovery rate: a practical and powerful approach to multiple testing. J Roy Stat Soc B 57(1):289–300
Lynch HT, Drescher KM, de la Chapelle A (2008) Immunology and the Lynch syndrome. Gastroenterology 134(4):1246–1249. https://doi.org/10.1053/j.gastro.2008.02.008
Klatka J, Grywalska E, Hymos A, Krasowska E, Mielnik M, Siwicka-Gieroba D, Markowicz J, Trojanowski P, Olszanski W, Rolinski J (2017) Subpopulations of natural killer-T-like cells before and after surgical treatment of laryngeal cancer. Cent Eur J Immunol 42(3):252–258. https://doi.org/10.5114/ceji.2017.70967
Grimm M, Feyen O, Hofmann H, Teriete P, Biegner T, Munz A, Reinert S (2016) Immunophenotyping of patients with oral squamous cell carcinoma in peripheral blood and associated tumor tissue. Tumour Biol 37(3):3807–3816. https://doi.org/10.1007/s13277-015-4224-2
Angka L, Martel AB, Kilgour M, Jeong A, Sadiq M, de Souza CT, Baker L, Kennedy MA, Kekre N, Auer RC (2018) Natural killer cell IFNgamma secretion is profoundly suppressed following colorectal cancer surgery. Ann Surg Oncol 25(12):3747–3754. https://doi.org/10.1245/s10434-018-6691-3
Molling JW, Kolgen W, van der Vliet HJ, Boomsma MF, Kruizenga H, Smorenburg CH, Molenkamp BG, Langendijk JA, Leemans CR, von Blomberg BM, Scheper RJ, van den Eertwegh AJ (2005) Peripheral blood IFN-gamma-secreting Valpha24+Vbeta11+ NKT cell numbers are decreased in cancer patients independent of tumor type or tumor load. Int J Cancer 116(1):87–93. https://doi.org/10.1002/ijc.20998
Dovsak T, Ihan A, Didanovic V, Kansky A, Verdenik M, Hren NI (2018) Effect of surgery and radiotherapy on complete blood count, lymphocyte subsets and inflammatory response in patients with advanced oral cancer. BMC Cancer 18(1):235. https://doi.org/10.1186/s12885-018-4136-9
Wang WH, Xu HY, Zhao ZM, Zhang GM, Lin FW (2018) Dynamic and significant changes of T-cell subgroups in breast cancer patients during surgery and chemotherapy. Int Immunopharmacol 65:279–283. https://doi.org/10.1016/j.intimp.2018.09.039
Lachmann G, von Haefen C, Kurth J, Yuerek F, Spies C (2018) Innate immunity recovers earlier than acquired immunity during severe postoperative immunosuppression. Int J Med Sci 15(1):1–9. https://doi.org/10.7150/ijms.21433
Verma R, Foster RE, Horgan K, Mounsey K, Nixon H, Smalle N, Hughes TA, Carter CR (2016) Lymphocyte depletion and repopulation after chemotherapy for primary breast cancer. Breast Cancer Res 18(1):10. https://doi.org/10.1186/s13058-015-0669-x
Acknowledgements
We thank Ronald van Vlierberghe, Rob Keijzer, Geeske Dekker-Ensink (Department of Surgery, LUMC) and Camilla Darum Sørensen (Department of Biomedicine, Aarhus University), for their contribution to this study. Specifically, we thank the FACS Core Facility of Aarhus University for their help and advice regarding the set-up of protocols and analyzing data in flow cytometry, and Nelleke Duinkerken (Department of Hematology, LUMC) for providing PBMC samples from healthy donors.
Funding
This project was supported by grants from the European Commission (Erasmus Plus Programme, ET 2020), Leiden University Fund (LUF International Study Fund (LISF)), Leiden University Medical Center (DOO Internationalization scholarship) and The Cancer Foundation (Marianne Hokland) and from the Prof E.L. Noach award (Natasja L. de Vries).
Author information
Authors and Affiliations
Contributions
DK, NLDV, AS, MN, MH and PJKK designed the experiments. AS contributed to sample preparation. DK and NLD carried out the experiments. DK analyzed the data and wrote the manuscript. EB verified the statistical methods. All authors provided critical feedback and contributed to the final manuscript. MH and PJKK supervised the project.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical standards.
All materials were obtained after approval by the Medical Ethical Committee of the LUMC (P000.193). All procedures performed in this study were in accordance with the ethical standards of the Dutch law (“WMO”, medical research involving human subjects act), the Danish law (“LBK” NR. 1083, 15 September 2017), and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
All CC patients included in this study agreed to the use of their PBMCs and data for research purposes prior to blood sampling via written informed consent. All CC patients agreed to anonymous publication of the resulting data.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Krijgsman, D., De Vries, N.L., Andersen, M.N. et al. The effects of tumor resection and adjuvant therapy on the peripheral blood immune cell profile in patients with colon carcinoma. Cancer Immunol Immunother 69, 2009–2020 (2020). https://doi.org/10.1007/s00262-020-02590-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-020-02590-z